Novel Schiff Bases of C-Methylresorcinarene Derivatives
Abstract
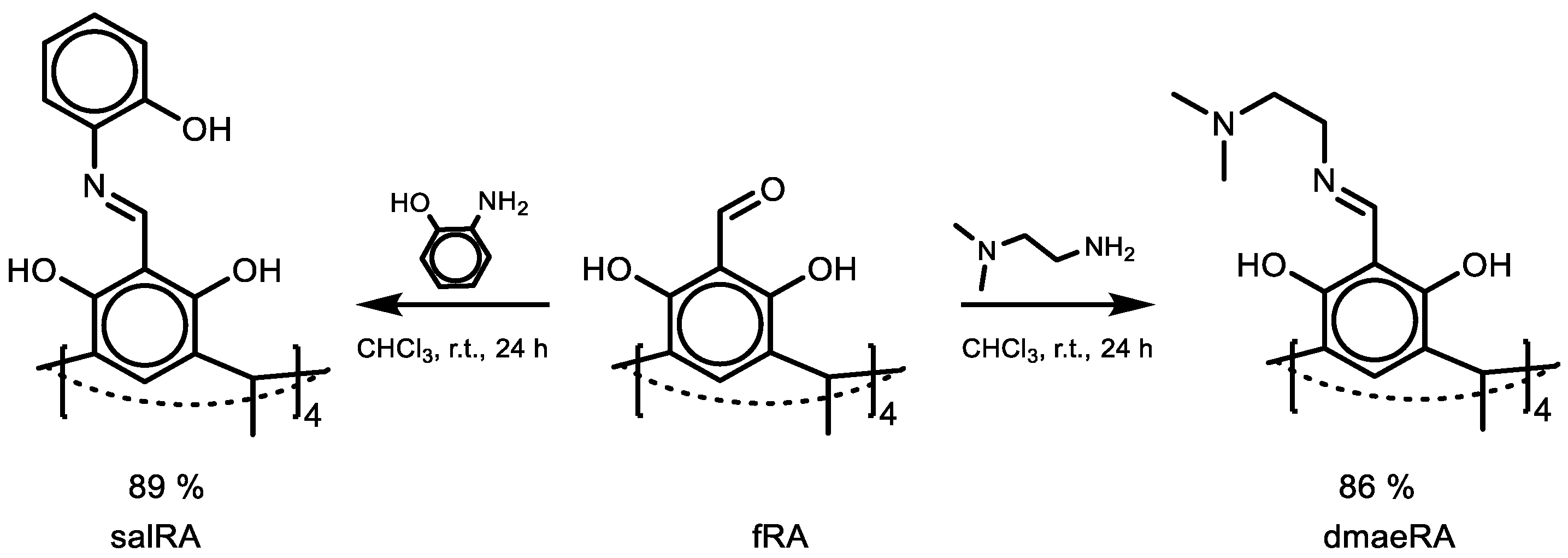
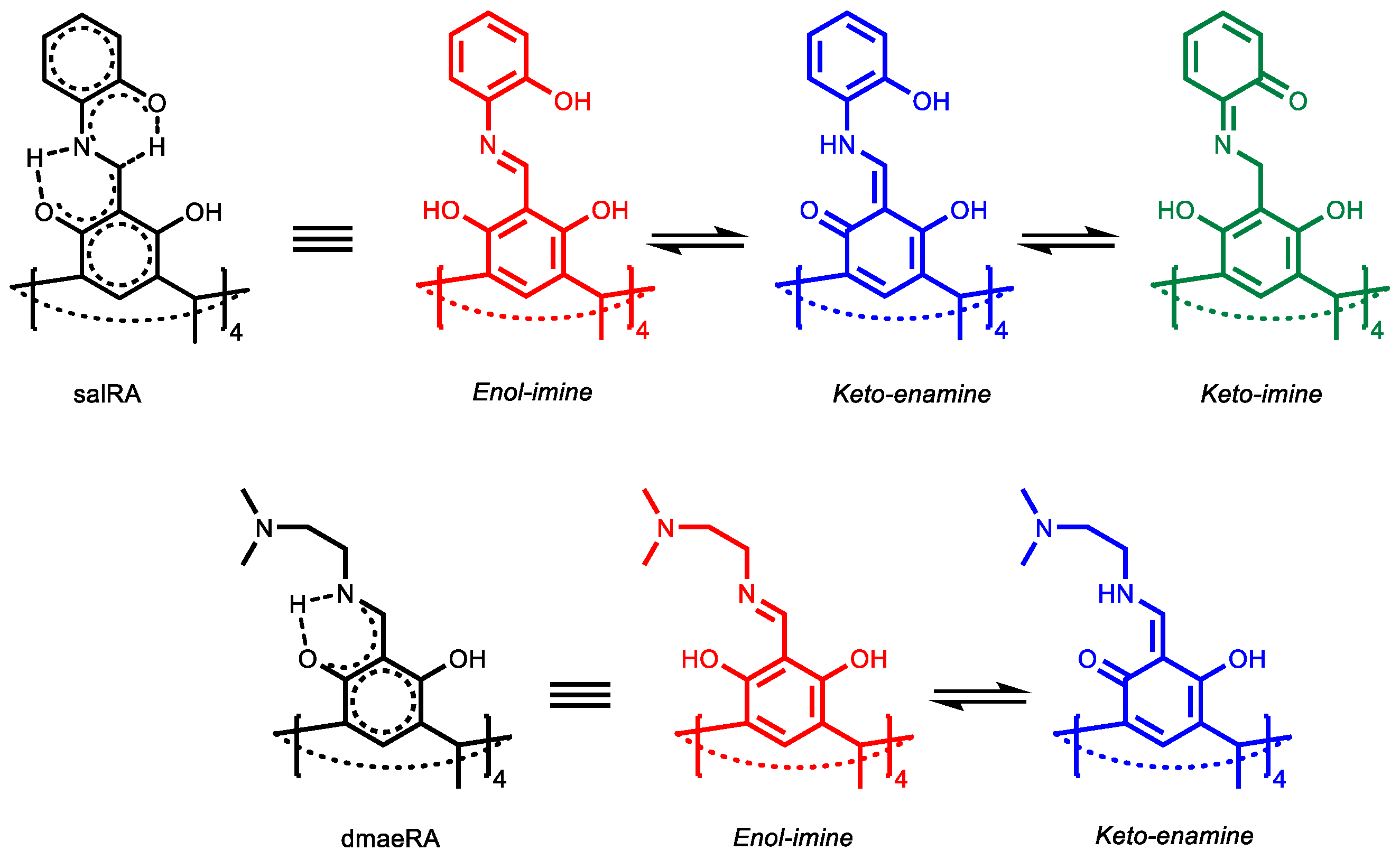
1. Introduction
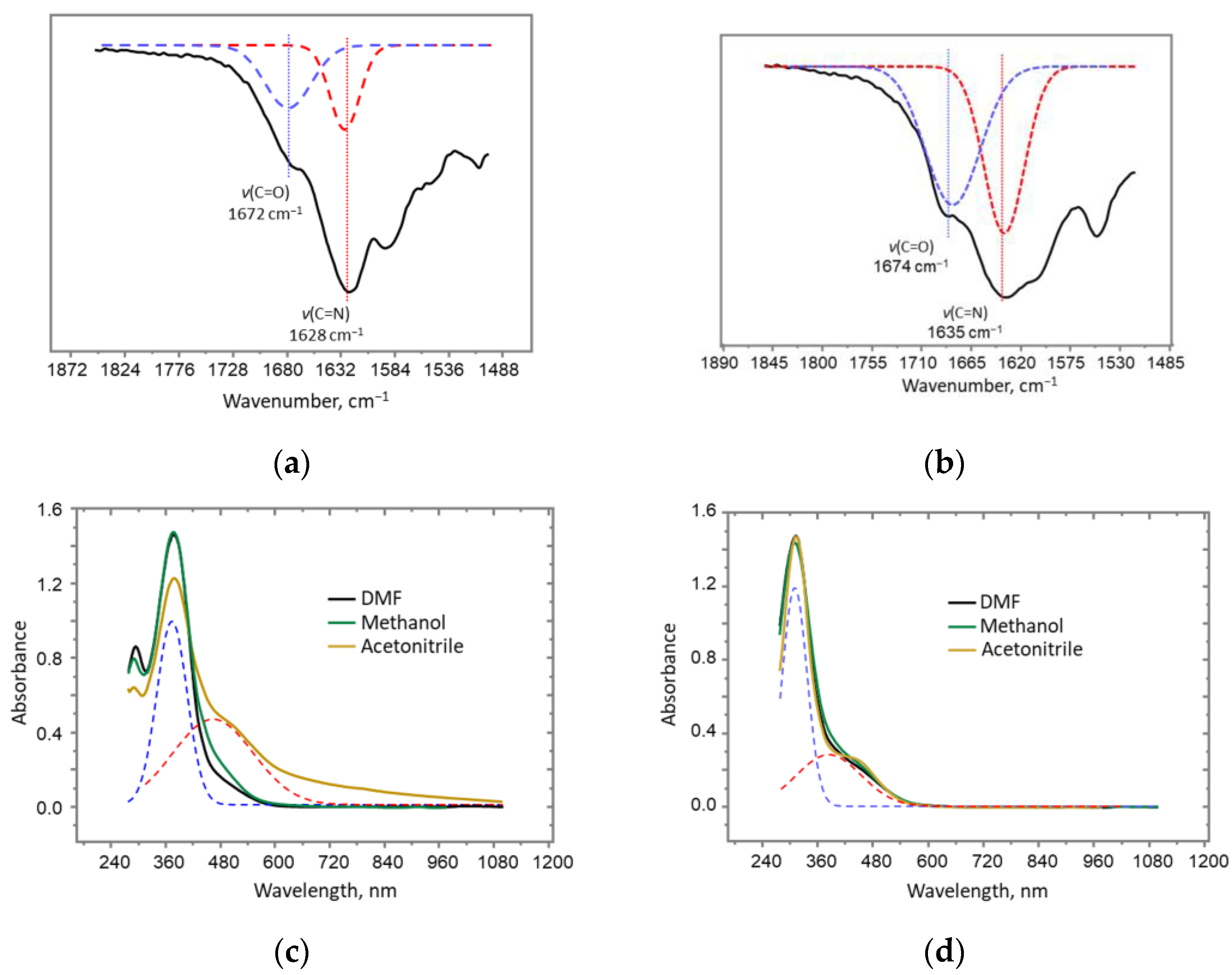
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhar, D.N.; Taplo, C.L. Schiff bases and their applications. J. Sci. Ind. Res. 1982, 41, 501–506. [Google Scholar]
- Przybylski, P.; Huczynski, A.; Pyta, K.; Brzezinski, B.; Bartl, F. Biological properties of Schiff bases and azo derivatives of phenols. Curr. Org. Chem. 2009, 13, 124–148. [Google Scholar] [CrossRef]
- Omidi, S.; Kakanejadifard, A. A review on biological activities of Schiff base, hydrazone, and oxime derivatives of curcumin. RSC Adv. 2020, 10, 30186–30202. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Hernández-Molina, R.; Mederos, A. Acyclic and macrocyclic Schiff base ligands in comprehensive coordination chemistry ii: From biology to nanotechnology. In Comprehensive Coordination Chemistry II: From Biology to Nanotechnology, 2nd ed.; McCleverty, J.A., Meyer, T.J., Eds.; Pergamon: London, UK, 2003; Volume 1, pp. 411–446. [Google Scholar]
- Gajjar, J.A.; Vekariya, R.H.; Sharma, V.S.; Kher, S.N.; Rajani, D.P.; Parekh, H.M. Mesomorphic properties, microwave-assisted synthesis, and antimicrobial evaluation of novel Schiff base functionalized resorcin [4] arene derivatives. Mol. Cryst. Liq. Cryst. 2021, 715, 37–55. [Google Scholar] [CrossRef]
- Jayswal, K.P.; Patel, J.R.; Patel, V.B.; Patel, M.H. A new approach towards synthesis of some novel “upper rim” functionalized calix[4]resorcinarene Schiff-bases. Acta Chim. Slov. 2008, 55, 302–307. [Google Scholar]
- Ge, Y.; Yan, C. Rapid synthesis of calix[4]resorcinarene-based dendrimers containing salicylideneimine terminal groups. J. Chem. Res. 2004, 2004, 279–281. [Google Scholar] [CrossRef]
- Vicens, J.; Harrowfield, J.; Baklouti, L. Calixarenes in the Nanoworld; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Upadhyay, J.B.; Parekh, H.M. Resorcin[4]arene Schiff base derivatives: Synthesis, characterization, and extraction studies. J. Chem. Res. 2020, 44, 660–666. [Google Scholar] [CrossRef]
- Neri, P.; Sessler, J.L.; Wang, M.-X. Calixarenes and Beyond; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Baldini, L.; Casnati, A.; Sansone, F.; Ungaro, R. Calixarene-based multivalent ligands. Chem. Soc. Rev. 2007, 36, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Quang, D.T. Calixarene-derived fluorescent probes. Chem. Rev. 2007, 107, 3780–3799. [Google Scholar] [CrossRef]
- Uddin, M.N. Biomedical applications of Schiff base metal complexes. J. Coord. Chem. 2020, 73, 3109–3149. [Google Scholar] [CrossRef]
- Catalano, A.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scali, E.; Saturnino, C.; Longo, P. A review on the advancements in the field of metal complexes with schiff bases as antiproliferative agents. Appl. Sci. 2021, 11, 6027. [Google Scholar] [CrossRef]
- Creaven, B.S.; Donlon, D.F.; McGinley, J. Coordination chemistry of calix[4]arene derivatives with lower rim functionalisation and their applications. Coord. Chem. Rev. 2009, 253, 893–962. [Google Scholar] [CrossRef]
- Eichstaedt, K.; Szpotkowski, K.; Grajda, M.; Gilski, M.; Wosicki, S.; Jaskjlski, M.; Szumna, A. Self-assembly and ordering of peptide-based cavitands in water and DMSO: The power of hydrophobic effects combined with neutral hydrogen bonds. Chem. Eur. J. 2019, 25, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Pizzala, H.; Carles, M.; Stone, W.E.E.; Thevand, A. Tautomerism in Schiff bases derived from 3-hydroxysalicylaldehyde. Combined X-ray diffraction, solution and solid state NMR study. J. Chem. Soc. Perkin Trans. 2000, 2, 935–939. [Google Scholar] [CrossRef]
- Dobosz, R.; Mućko, J.; Gawinecki, R. Using Chou’s 5-step rule to evaluate the stability of tautomers: Susceptibility of 2-[(phenylimino)-methyl]-cyclohexane-1,3-diones to tautomerization based on the calculated Gibbs free energies. Energies 2020, 13, 183. [Google Scholar] [CrossRef]
- Martínez, R.F.; Ávalos, M.; Babiano, R.; Cintas, P.; Jiménez, J.L.; Light, M.E.; Palacios, J.C. Tautomerism in Schiff bases. The cases of 2-hydroxy-1-naphthaldehyde and 1-hydroxy-2-naphthaldehyde investigated in solution and the solid state. Org. Biomol. Chem. 2011, 9, 8268–8275. [Google Scholar] [CrossRef]
- Grajda, M.; Wierzbicki, M.; Cmoch, P.; Szumna, A. Inherently Chiral Iminoresorcinarenes through Regioselective Unidirectional Tautomerization. J. Org. Chem. 2013, 78, 11597–11601. [Google Scholar] [CrossRef]
- Jędrzejewska, H.; Kwit, M.; Szumna, A. Switching of inherent chirality driven by self-assembly. Chem. Commun. 2015, 51, 13799–13801. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.R.; Saleh, N.A.I. Infra-red study of tautomerism in some Schiff bases. Spectrosc. Lett. 1997, 30, 1289–1300. [Google Scholar] [CrossRef]
- Atzin-Macedo, C.M.; Pastor-Ramírez, C.; González-Peláez, R.; Pérez-Flores, F.J.; Hernández-Anzaldo, S.; Vazquez-Lima, H.; Reyes-Ortega, Y. Tautomeric study of Schiff bases derived from o-dihydroxybenzaldehyde by UV-Vis, IR, 1H NMR, 13C NMR spectroscopy and computational modeling. ChemistrySelect 2020, 5, 11120–11126. [Google Scholar] [CrossRef]
- Minkin, V.I.; Tsukanov, A.V.; Dubonosov, A.D.; Bren, V.A. Tautomeric Schiff bases: Iono-, solvato-, thermo- and photochromism. J. Mol. Struct. 2011, 998, 179–191. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, A.Y.; Saranova, O.S.; Fazleeva, R.R.; Yanilkin, V.V.; Antipin, I.S. Novel Schiff Bases of C-Methylresorcinarene Derivatives. Molbank 2022, 2022, M1505. https://doi.org/10.3390/M1505
Ziganshina AY, Saranova OS, Fazleeva RR, Yanilkin VV, Antipin IS. Novel Schiff Bases of C-Methylresorcinarene Derivatives. Molbank. 2022; 2022(4):M1505. https://doi.org/10.3390/M1505
Chicago/Turabian StyleZiganshina, Albina Y., Olga S. Saranova, Rezeda R. Fazleeva, Vitaly V. Yanilkin, and Igor S. Antipin. 2022. "Novel Schiff Bases of C-Methylresorcinarene Derivatives" Molbank 2022, no. 4: M1505. https://doi.org/10.3390/M1505
APA StyleZiganshina, A. Y., Saranova, O. S., Fazleeva, R. R., Yanilkin, V. V., & Antipin, I. S. (2022). Novel Schiff Bases of C-Methylresorcinarene Derivatives. Molbank, 2022(4), M1505. https://doi.org/10.3390/M1505





