Abstract
Betulin has a broad spectrum of biological and pharmacological properties, such as anticancer, antibacterial, antifungal, and antiviral. Unfortunately, the low bioavailability makes it difficult to use in medicine. The introduction of a triazole ring to the betulin structure leads to the obtainment of new compounds with higher activity and better bioavailability. The title compound was obtained from the triazole derivative of betulin by conversion of the hydroxyl group to an ester moiety in the Steglich reaction. The chemical structure of the hybrid was characterized by nuclear magnetic resonance (1H NMR, 13C NMR, HSQC, HMBC) and HRMS spectroscopy.
1. Introduction
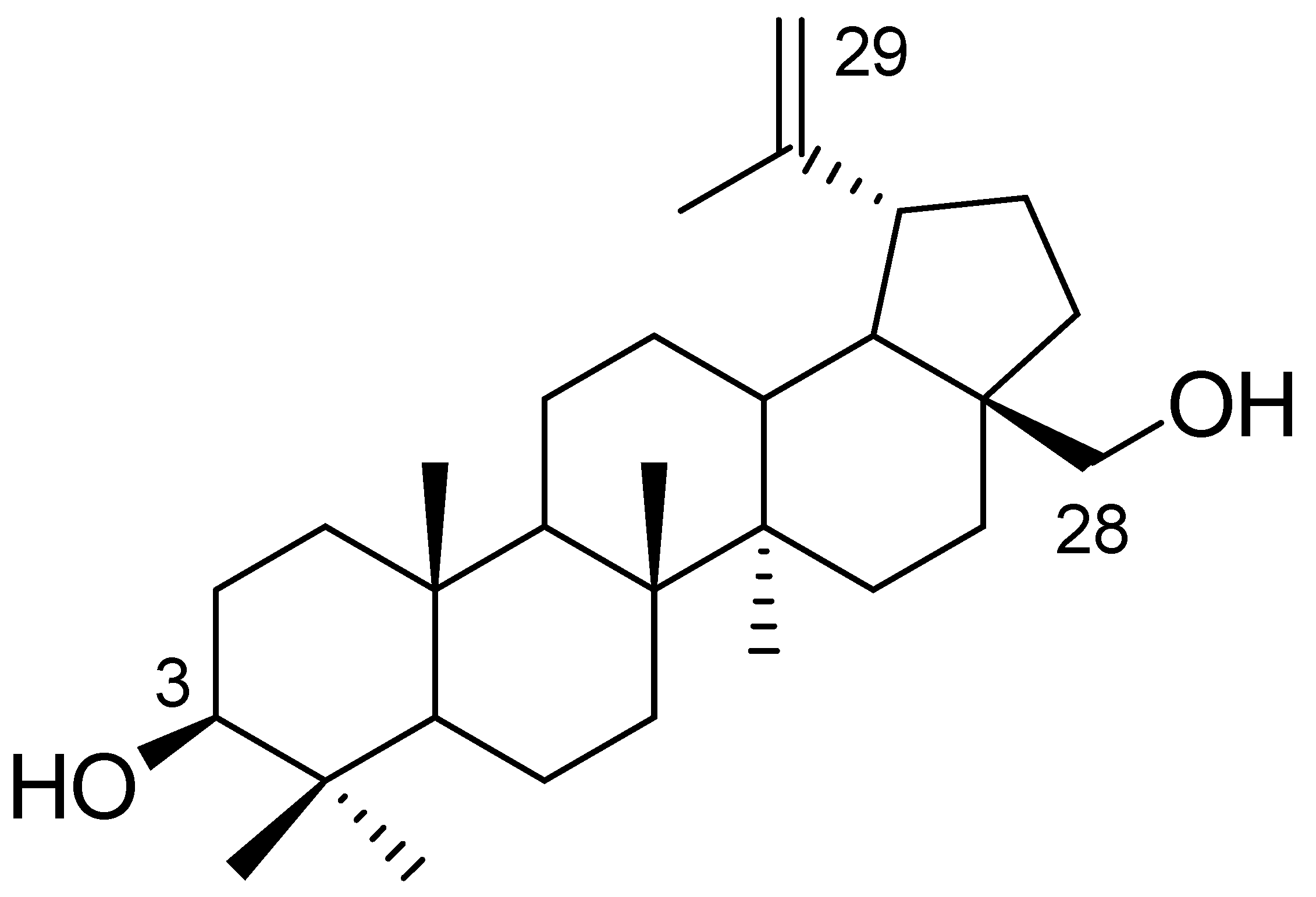
Betulin, a pentacyclic triterpene of the lupane-type, has a wide range of biological and pharmacological properties. This compound is characterized by poor water solubility, which reduces its bioavailability. Betulin can be used as a building block for the synthesis of new derivatives by converting the hydroxyl groups at the C3 and C28 positions or by introducing modifications to the isopropenyl group attached to a five-membered ring (Figure 1) [1,2,3,4,5,6].
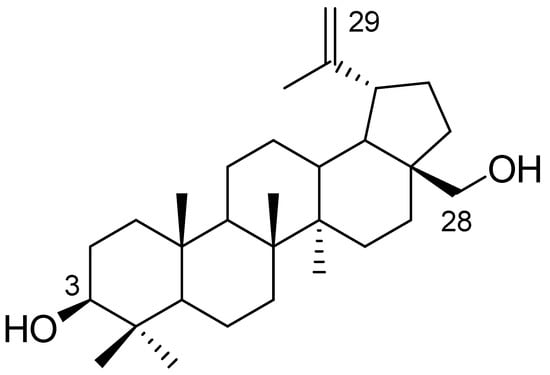
Figure 1.
Chemical structure of betulin.
Betulin undergoes reactions characteristic of alcohols, such as esterification and oxidation [7]. In the synthesis of betulin esters, Steglich esterification is usually used. This reaction takes place under mild conditions and does not require high temperatures. The synthesis of esters by this method is performed with N,N’-dicyclohexylcarbodiimide (DCC), and 4-(dimethylamino)pyridine (DMAP). The addition of DMAP accelerates the reaction and reduces the formation of by-products associated with the migration of the acyl group [8,9].
The modification of betulin is also associated with the introduction of the triazole ring. The triazole ring occurs in the chemical structure of compounds that exhibit anticancer, anti-inflammatory, anti-tuberculosis, antibacterial, antifungal, antioxidant, and analgesic effects. The possibility of introducing various substituents into the triazole ring is important for modulating the biological properties of the triazoles and allows their wide application [10].
It is supposed that hybrid compounds can reduce side effects and overcome drug resistance. Hybrids with several pharmacophores may also exhibit different mechanisms of biological action. The combination of the triazole system with other anticancer pharmacophores may lead to the formation of new derivatives of low toxicity and greater effectiveness in the treatment of drug-resistant neoplasms [11]. For example, betulin-triazole hybrids often possess higher biological activity than betulin. It has been shown that triazole derivatives of betulin are cytotoxic to cells of lymphoblastic leukemia, cervical cancer, ovarian cancer, breast cancer, colon cancer, prostate cancer, lung cancer, and melanoma [12,13,14,15,16]. Moreover, in the group of triazole derivatives of pentacyclic triterpenes, there are compounds with antimicrobial, antiviral, and neuroprotective effects [17,18,19].
The study describes the synthesis of new triazole derivatives of betulin. The structure of the title compound was characterized by homo- (1H and 13C NMR) and heteronuclear (HSQC and HMBC) magnetic resonance spectroscopy and HRMS spectrometry.
2. Results and Discussion
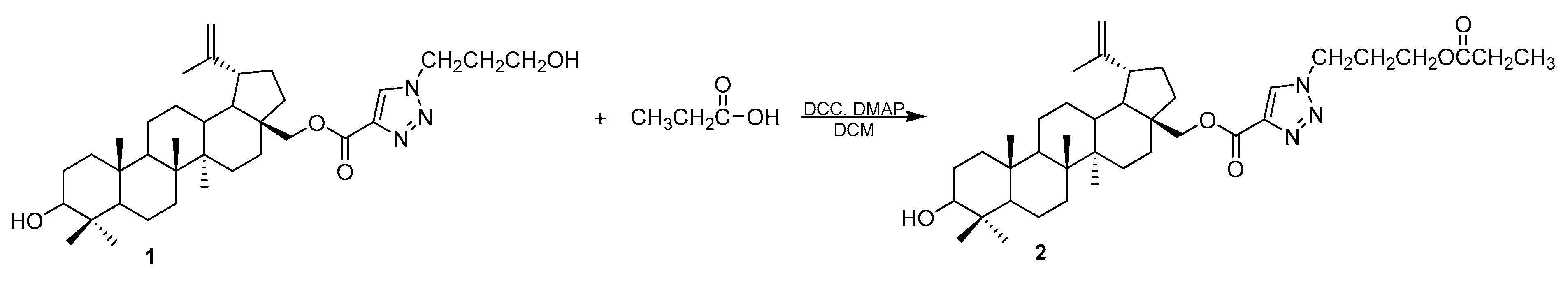
The triazole derivative 1 was synthesized from betulin in a multi-step reaction which is described in our earlier papers [13,16]. The treatment of compound 1 with propanoic acid in the presence of N,N’-dicyclohexylcarbodiimide (DCC), and 4-dimethylaminopyridine (DMAP) in dichloromethane (DCM) leads to ester 2 (Scheme 1).
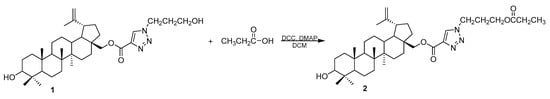
Scheme 1.
Synthesis of 28-[1-(3-(propionyloxy)propyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin 2.
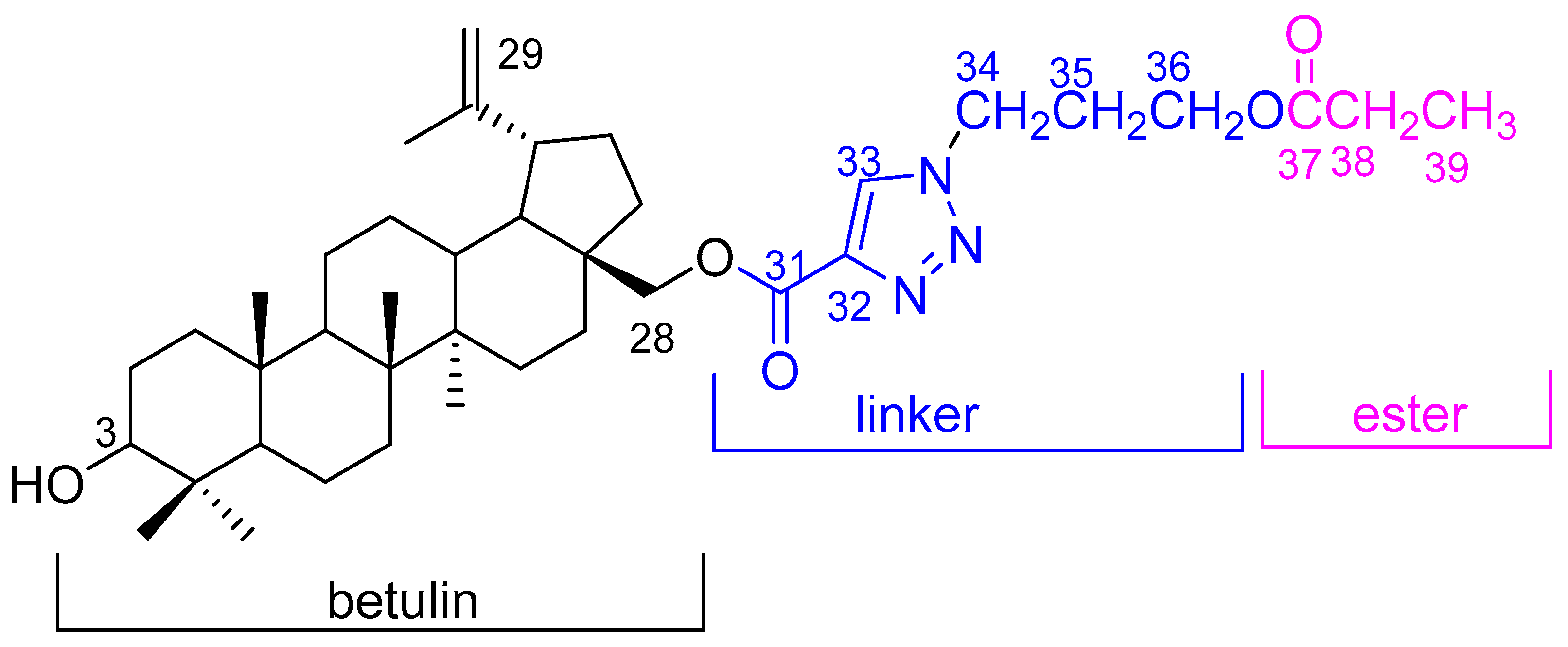
The crude product was purified by column chromatography. Compound 2 was obtained with a high yield (79%). The structure of the title compound 2, which consists of three moieties, betulin, triazole linker, and the ester group (Figure 2), was characterized by 1D (1H and 13C) and 2D (HMBC and HSQC) NMR and the HR-MS spectra.
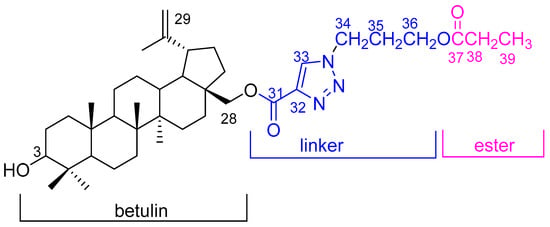
Figure 2.
Chemical structure of compound 2.
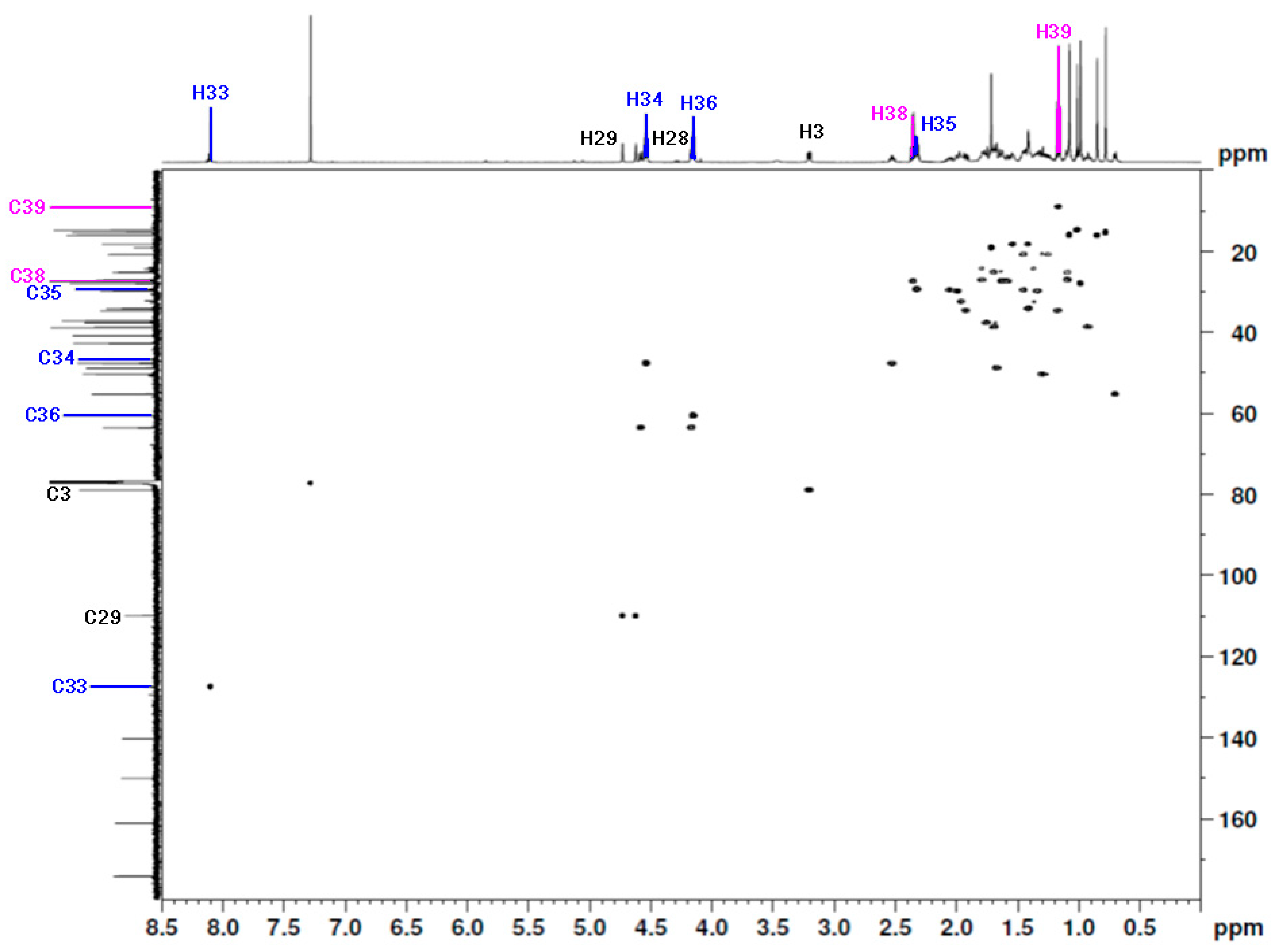
The chemical shift of the main hydrogen and carbon atoms in the betulin moiety was assigned based on the literature data [20,21]. The 1H NMR spectra of the betulin scaffold indicated the presence of six methyl groups at δH 0.78, 0.85, 0.99, 1.01, 1.08, and 1.72 ppm. The chemical shift of the proton at the C3 position was observed at δH 3.21 ppm. Signals located at δH 4.15 ppm and 4.59 ppm were assigned to the protons of the methylene group in position C28. The singlets at δH 4.62 ppm and 4.73 ppm indicated the presence of protons at a C29 position (Table 1, Figure 3). In the 13C NMR spectrum of the title compound, the signal at δC 79.0 ppm was assigned to the C3 carbon atom, which is characteristic of betulin derivatives containing a hydroxyl group in this position. The signals at δC 63.6 ppm and 110.0 ppm were assigned to carbon atoms at C28 and C29, respectively (Table 1, Figure 3).

Table 1.
The selected chemical shifts (1H NMR and 13C NMR spectra) and correlations of proton–carbon (HSQC and HMBC experiments) for derivative 2.
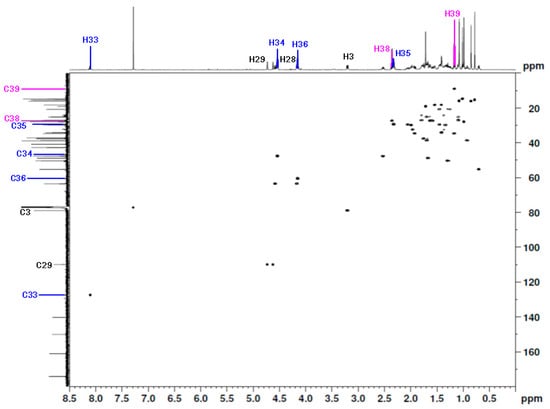
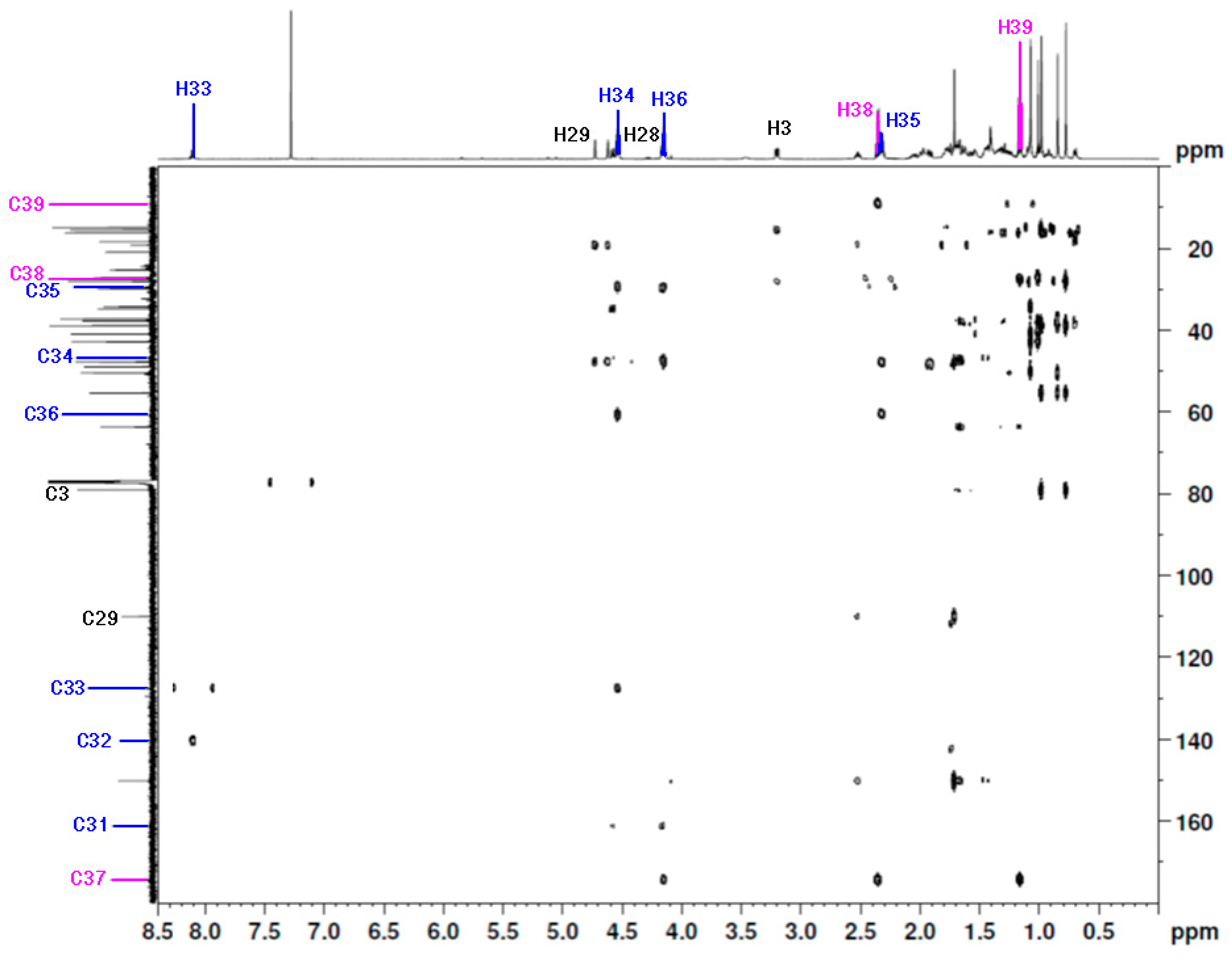
Figure 3.
The 1H-13C HSQC spectrum (600 MHz, CDCl3) of 28-[1-(3-(propionyloxy)propyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin 2.
The signals of the triazole linker and the ester moiety were assigned on the basis of the HMBC correlation spectra (Table 1, Figure 4). The HMBC shows that the methyl group at the C39 position correlated with C38 and C37. The carbon atoms at the C37 and C39 positions were identified based on their correlation with the proton at the C38 position. Furthermore, the C37 carbon (δC 174.2 ppm) was correlated with the H39, H38, and H36 protons, respectively. The spectrum also showed the correlation of CH2 in position C34 with the carbon atom in position C35 (δC 29.4 ppm) and C33 (δC 127.5 ppm), respectively. The correlation between the proton signal at C33 (δC 8.10 ppm) and the carbon signal at C32 (δC 140.3 ppm) was also observed. The carbonyl group at the C31 position had a correlation with the carbon atom at the C28 (δC 63.6 ppm) position (Table 1, Figure 4).
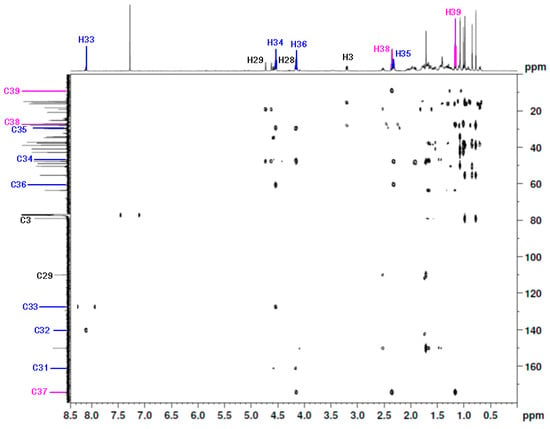
Figure 4.
The 1H-13C HMBC spectrum (600 MHz, CDCl3) of 28-[1-(3-(propionyloxy)propyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin 2.
The exact mass of the [M + Na]+ ion, determined by ESI-HRMS, was found to be 674.4505 (674.4509 as calculated for C39H61N3O5Na+).
3. Materials and Methods
3.1. General Method
All reagents were purchased from Sigma-Aldrich (Darmstadt, Germany). The 28-[1-(3-hydroxypropyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin 1 was obtained using the literature method [16]. The 1H and 13C NMR spectra were acquired on the Bruker Avance 600 spectrometer (Brucker Analytische Messtechnik GmbH, Rheinstetten, Germany) at 600 MHz and 150 MHz, respectively, as well as the HMBC and HSQC NMR spectra. The compound was dissolved in a deuterated chloroform (CDCl3) solvent. Chemical shifts (δ) were reported in ppm and J values in Hz. Multiplicity was designated as singlet (s), doublet (d), triplet (t), and multiplet (m). The protons of betulin, the triazole linker, and the ester moiety were denoted by the appropriate indices as beta, linker, and ester. High-resolution mass spectra were measured on the Bruker Impact II instrument (Brucker Analytische Messtechnik GmbH, Rheinstetten, Germany). Melting points were measured by the Electrothermal IA 9300 melting point apparatus.
3.2. Synthesis of 28-[1-(3-(Propionyloxy)propyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin 2
The 28-[1-(3-hydroxypropyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin 1 0.60 g (1 mmol) and propanoic acid 84 µL (0.310 mmol, 0.08 g) were dissolved in 4 mL of dichloromethane (DCM) and cooled to −10 °C. At this temperature, the mixture of 0.116 g DCC (0.605 mmol) and DMAP 0.005 g (0.080 mmol) in the dichloromethane (1 mL) was dropped. The reaction mixture was stirred overnight at room temperature. Then, the precipitate was filtered off, and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography (CHCl3:EtOH, 40:1, v/v). The desired product was a white crystalline solid (mp. 230–232 °C, yield 79%, 0.516 g, 0.79 mmol).
1H NMR (600 MHz, CDCl3) δ, ppm: 0.65 (d, J = 9.36 Hz, 1H, H5bet), 0.78 (s, 3H, CH3bet), 0.85 (s, 3H, CH3bet), 0.99 (s, 3H, CH3bet), 1.01 (s, 3H, CH3bet), 1.08 (s, 3H, CH3bet), 1.16 (t, J = 7.56 Hz, 3H, CH3ester), 1.72 (s, 3H, CH3bet), 0.90–2.09 (m, 25H, CHbet, CH2bet), 2.32 (m, 2H, CH2linker); 2.36 (m, CH2ester), 2.53 (m, 1H, H19bet), 3.21 (m, 1H, H3bet), 4.15 (m, 3H, CH2linker, H28bet), 4.54 (t, J = 6.96 Hz, 2H, CH2linker); 4.59 (d, J = 10.8 Hz, 1H, H28bet), 4.62 (s, 1H, H29bet), 4.73 (s, 1H, H29bet), 8.10 (s, 1H, CHlinker) (Figure S1), 13C NMR (150 MHz, CDCl3) δ, ppm: 9.1, 14.8, 15.4, 16.1, 18.3, 20.8, 24.3, 27.1, 27.4, 28.0, 29.4, 29.6, 29.8, 34.2, 34.7, 37.2, 37.7, 38.7, 38.9, 40.9, 42.8, 46.7, 47.7, 48.9, 50.4, 55.3, 60.6, 63.6, 79.0, 110.0, 127.5, 129.5, 140.3, 150.1, 161.1, 174.2 (Figure S2). ESI-HRMS m/z [M + Na]+ calcd for C39H61N3O5Na+ 674.4509, found 674.4505 (Figure S3).
Supplementary Materials
The following supporting information are available online, Figure S1: 1H NMR spectrum (600 MHz, CDCl3) of 28-[1-(3-(propionyloxy)propyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin; Figure S2: 13C NMR spectrum (150 MHz, CDCl3) of 28-[1-(3-(propionyloxy)propyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin; Figure S3: ESI-HRMS spectrum of 28-[1-(3-(Propionyloxy)propyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin.
Author Contributions
Conceptualization, methodology, writing—original draft preparation, M.K.-T.; formal analysis, writing—original draft preparation, E.C. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Silesia in Katowice, Poland. Grants No PCN-1-042/K/2/F and PCN-1-044/K/2/F.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaur, P.; Arora, S.; Singh, R. Isolation, characterization and biological activities of betulin from Acacia nilotica bark. Sci. Rep. 2022, 12, 9370. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Szewczyk, A.; Galanty, A.; Gdula-Argasińska, J.; Muszyńska, B. Chemical composition and biological activity of extracts from fruiting bodies and mycelial cultures of Fomitopsis betulina. Mol. Biol. Rep. 2018, 45, 2535–2544. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Pavlova, N.I.; Savinova, O.V.; Nikolaeva, S.N.; Boreko, E.; Flekhterb, I.B. Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses. Fitoterapia 2003, 75, 489–492. [Google Scholar] [CrossRef]
- Oloyede, O.H.B.; Ajiboye, H.O.; Salawu, M.; Ajiboye, O.T. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Myz, S.A.; Mikhailovskaya, A.V.; Mikhailenko, M.A.; Bulina, M.; Kuznetsova, S.; Shakhtshneider, T.P. New crystalline betulin-based materials: Improving betulin solubility via cocrystal formation. Mater. Today: Proc. 2019, 12, 82–85. [Google Scholar] [CrossRef]
- Grigoreva, A.; Kolobova, E.; Pakrieva, E.; Mäki-Arvela, P.; Kuznetsova, S.; Carabineiro, S.A.C.; Bogdanchikova, N.; Pestryakov, A.; Murzin, D.Y. Liquid-phase oxidation of betulin over supported Ag NPs catalysts: Kinetic regularities, catalyst deactivation and reactivation. Mol. Catal. 2022, 528, 112461. [Google Scholar] [CrossRef]
- Asmaa, M.F.; Amr, M.; Medhat, A.I. Experimental and theoretical studies of some propiolate esters derivatives. J. Mol. Struct. 2021, 1236, 130281. [Google Scholar]
- Boryczka, S.; Bębenek, E.; Wietrzyk, J.; Kempińska, K.; Jastrzębska, M.; Kusz, J.; Nowak, M. Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules 2013, 18, 4526–4543. [Google Scholar] [CrossRef]
- Abdelli, A.; Azzouni, S.; Plais, R.; Gaucher, A.; Efrit, M.L.; Prim, D. Recent advances in the chemistry of 1,2,4-triazoles: Synthesis, reactivity and biological activities. Tetrahedron Lett. 2021, 86, 153518. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, S.J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents:Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef] [PubMed]
- Kuczynska, K.; Bończak, B.; Rárová, L.; Kvasnicová, M.; Strnad, M.; Pakulski, Z.; Cmoch, P.; Fiałkowski, M. Synthesis and cytotoxic activity of 1,2,3-triazoles derived from 2,3-seco-dihydrobetulin via a click chemistry approach. J. Mol. Struct. 2022, 1250, 131751. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Marciniec, K.; Chrobak, E.; Bębenek, E.; Latocha, M.; Kuśmierz, D.; Boryczka, S. Design, synthesis and biological activity of 1,4-quinone moiety attached to betulin derivatives as potent DT-diaphorase substrate. Bioorg. Chem. 2021, 106, 104478. [Google Scholar] [CrossRef]
- Chrobak, E.; Kadela-Tomanek, M.; Bębenek, E.; Marciniec, K.; Wietrzyk, J.; Trynda, J.; Pawełczak, B.; Kusz, J.; Kasperczyk, J.; Chodurek, E.; et al. New phosphate derivatives of betulin as anticancer agents: Synthesis, crystal structure, and molecular docking study. Bioorg. Chem. 2019, 87, 613–628. [Google Scholar] [CrossRef]
- Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Latocha, M.; Boryczka, S. Novel triazoles of 3-acetylbetulin and betulone as anticancer agents. Med. Chem. Res. 2018, 27, 2051–2061. [Google Scholar] [CrossRef]
- Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Wietrzyk, J.; Sadowska, J.; Boryczka, S. New acetylenic derivatives of betulin and betulone, synthesis and cytotoxic activity. Med. Chem. Res. 2017, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Jastrzębska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel triazole hybrids of betulin: Synthesis and biological activity profile. Molecules 2017, 22, 1876–1898. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Hu, H.; Persoons, L.; Daelemans, D.; De Jonghe, S.; Luyten, W.; Krasniqi, B.; Dehaen, W. Antibacterial and antitumoral properties of 1,2,3-triazolo fused triterpenes and their mechanism of inhibiting the proliferation of HL-60 cells. Eur. J. Med. Chem. 2021, 224, 113727. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Shi, Y.; Si, L.; Jiao, P.; Fan, Z.; Han, H.; Wu, X.; Zhou, X.; Yu, F.; et al. Design, synthesis and biological evaluation of novel l-ascorbic acid-conjugated pentacyclic triterpene derivatives as potential influenza virus entry inhibitors. Eur. J. Med. Chem. 2016, 110, 376–388. [Google Scholar] [CrossRef]
- Gonzalez, G.; Hodoň, J.; Kazakova, A.; D’Acunto, C.; Kaňovský, P.; Urban, M.; Strnad, M. Novel pentacyclic triterpenes exhibiting strong neuroprotective activity in SH-SY5Y cells in salsolinol- and glutamate-induced neurodegeneration models. Eur. J. Med. Chem. 2021, 213, 113168. [Google Scholar] [CrossRef]
- Liao, C.R.; Kuo, Y.H.; Ho, Y.L.; Wang, C.Y.; Yang, C.S.; Lin, C.W.; Chang, Y.S. Studies on cytotoxic constituents from the leaves of Elaeagnus oldhamii Maxim. in non-small cell lung cancer A549 cells. Molecules 2014, 19, 9515–9534. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).