8,13-Dimethylicosa-9,11-diyne-8,13-diol
Abstract
1. Introduction
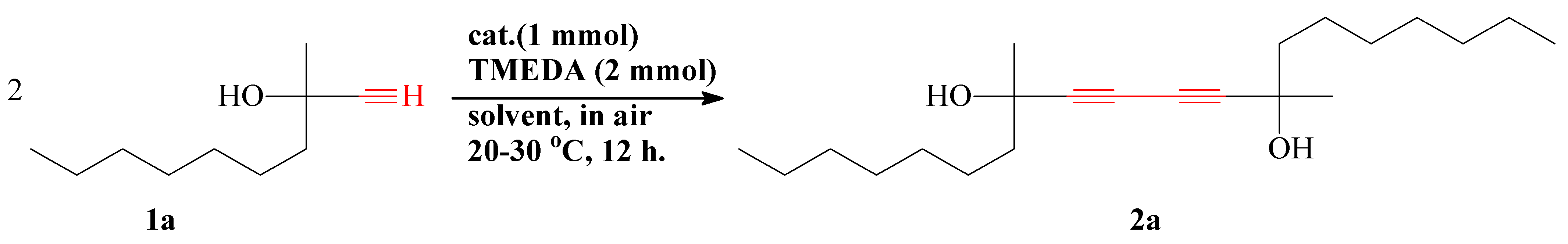
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of 2a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, R.K.; Liu, Y.; Xie, L. Reinforcement Learning for Systems Pharmacology-Oriented and Personalized Drug Design. Expert Opin. Drug Discov. 2022, 17, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-J.; Kwon, H.-S.; Kang, M.; Leem, H.; Lee, K.-H.; Kim, D.-Y. The Antitumor Natural Compound Falcarindiol Disrupts Neural Stem Cell Homeostasis by Suppressing Notch Pathway. Int. J. Mol. Sci. 2018, 19, 3432. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Isolobetyol, a New Polyacetylene Derivative from Platycodon grandiflorum Root. Nat. Prod. Res. 2022, 36, 466–469. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Yang, W.-C.; Liang, C.-L.; Liu, H.-Y.; Lai, S.-K.; Chang, C.L.-T. Cytopiloyne, a Polyacetylenic Glucoside from Bidens Pilosa, Acts as a Novel Anticandidal Agent via Regulation of Macrophages. J. Ethnopharmacol. 2016, 184, 72–80. [Google Scholar] [CrossRef]
- Geng, C.-A.; Huang, X.-Y.; Chen, X.-L.; Ma, Y.-B.; Rong, G.-Q.; Zhao, Y.; Zhang, X.-M.; Chen, J.-J. Three New Anti-HBV Active Constituents from the Traditional Chinese Herb of Yin-Chen (Artemisia scoparia). J. Ethnopharmacol. 2015, 176, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Fois, B.; Bianco, G.; Sonar, V.P.; Distinto, S.; Maccioni, E.; Meleddu, R.; Melis, C.; Marras, L.; Pompei, R.; Floris, C.; et al. Phenylpropenoids from Bupleurum fruticosum as Anti-Human Rhinovirus Species A Selective Capsid Binders. J. Nat. Prod. 2017, 80, 2799–2806. [Google Scholar] [CrossRef]
- Liu, X.; Latkolik, S.; Atanasov, A.; Kunert, O.; Pferschy-Wenzig, E.-M.; Heiss, E.; Malainer, C.; Schinkovitz, A.; Kollroser, M.; Dirsch, V.; et al. Bupleurum Chinense Roots: A Bioactivity-Guided Approach toward Saponin-Type NF-ΚB Inhibitors. Planta Med. 2017, 83, 1242–1250. [Google Scholar] [CrossRef]
- Chan, G.G.; Koch, C.M.; Connors, L.H. Blood Proteomic Profiling in Inherited (ATTRm) and Acquired (ATTRwt) Forms of Transthyretin-Associated Cardiac Amyloidosis. J. Proteome Res. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Xu, W.-J.; Li, J.-H.; Zhou, M.-M.; Luo, J.; Jian, K.-L.; Tian, X.-M.; Xia, Y.-Z.; Yang, L.; Luo, J.; Kong, L.-Y. Toonasindiynes A-F, New Polyacetylenes from Toona Sinensis with Cytotoxic and Anti-Inflammatory Activities. Fitoterapia 2020, 146, 104667. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Matsuura, D.; Kanatani, H.; Yano, S.; Tsunakawa, M.; Matsuyama, S.; Shigemori, H. Inhibitory Effects of Polyacetylene Compounds from Panax ginseng on Neurotrophin Receptor-Mediated Hair Growth. Biol. Pharm. Bull. 2017, 40, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-Y.; Yang, C.-T.; Pu, X.-Y.; Fu, G.; Wang, W.; Li, Y.-X.; Feng, L.; Niu, H.-R.; Tan, J.-L.; Huang, X.-Z. Polyacetylenes from the Roots of Aralia Dumetorum. Rec. Nat. Prod. 2019, 13, 424–428. [Google Scholar] [CrossRef]
- Siemsen, P.; Livingston, R.C.; Diederich, F. Acetylenic Coupling: A Powerful Tool in Molecular Construction. Angew. Chem. Int. Ed. 2000, 39, 2632–2657. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Reddy, K.B.; Gayathri, K.U.; Prasad, A.R. Glaser Oxidative Coupling in Ionic Liquids: An Improved Synthesis of Conjugated 1,3-Diynes. Tetrahedron Lett. 2003, 44, 6493–6496. [Google Scholar] [CrossRef]
- Liao, Y.; Fathi, R.; Yang, Z. Aliphatic Acetylenic Homocoupling Catalyzed by a Novel Combination of AgOTs−CuCl 2 −TMEDA and Its Application for the Solid-Phase Synthesis of Bis-Benzo[b]Furan-Linked 1,3-Diynes. Org. Lett. 2003, 5, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Atobe, S.; Sonoda, M.; Suzuki, Y.; Yamamoto, T.; Masuno, H.; Shinohara, H.; Ogawa, A. Palladium-Catalyzed Oxidative Homocoupling Reaction of Terminal Acetylenes Using Trans-BidentaTable 1-(2-Pyridylethynyl)-2-(2-Thienylethynyl)Benzene. Res. Chem. Intermed. 2013, 39, 359–370. [Google Scholar] [CrossRef]
- Zhu, B.C.; Jiang, X.Z. A New CuAl–Hydrotalcite Catalyzed Homocoupling Reaction of Terminal Alkynes at Room Temperature. Appl. Organomet. Chem. 2007, 21, 345–349. [Google Scholar] [CrossRef]
- Zheng, Q.; Hua, R.; Wan, Y. An Alternative CuCl–Piperidine-Catalyzed Oxidative Homocoupling of Terminal Alkynes Affording 1,3-Diynes in Air. Appl. Organomet. Chem. 2009, 24, 314–316. [Google Scholar] [CrossRef]
- Hosseini, A.; Seidel, D.; Miska, A.; Schreiner, P.R. Fluoride-Assisted Activation of Calcium Carbide: A Simple Method for the Ethynylation of Aldehydes and Ketones. Org. Lett. 2015, 17, 2808–2811. [Google Scholar] [CrossRef] [PubMed]
| Entry | Catalyst | Solvent | Additive | Time, h. | Yield, % a |
|---|---|---|---|---|---|
| 1 | CuCl | MeCN/CCl4 | TMEDA | 8 | trace b |
| 2 | CuCl | THF/CCl4 | TMEDA | 10 | 42 |
| 3 | CuCl | CH2OHCH2OH/CCl4 | TMEDA | 10 | 27 b |
| 4 | CuCl | iPrOH/CCl4 | TMEDA | 12 | 65 |
| 5 | CuCl | EtOH/CCl4 | TMEDA | 12 | 70 |
| 6 | CuCl | MeOH/CCl4 | TMEDA | 12 | 82 |
| 7 | CuCl | MeOH/CCl4 | 12 | trace b | |
| 8 | CuJ | MeOH/CCl4 | TMEDA | 12 | 64 |
| 9 | CuBr | MeOH/CCl4 | TMEDA | 12 | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirkasheva, S.I.; Ziyadullaev, O.E.; Muzalevskiy, V.M.; Parmanov, A.B. 8,13-Dimethylicosa-9,11-diyne-8,13-diol. Molbank 2022, 2022, M1484. https://doi.org/10.3390/M1484
Tirkasheva SI, Ziyadullaev OE, Muzalevskiy VM, Parmanov AB. 8,13-Dimethylicosa-9,11-diyne-8,13-diol. Molbank. 2022; 2022(4):M1484. https://doi.org/10.3390/M1484
Chicago/Turabian StyleTirkasheva, Sarvinoz I., Odiljon E. Ziyadullaev, Vasiliy M. Muzalevskiy, and Askar B. Parmanov. 2022. "8,13-Dimethylicosa-9,11-diyne-8,13-diol" Molbank 2022, no. 4: M1484. https://doi.org/10.3390/M1484
APA StyleTirkasheva, S. I., Ziyadullaev, O. E., Muzalevskiy, V. M., & Parmanov, A. B. (2022). 8,13-Dimethylicosa-9,11-diyne-8,13-diol. Molbank, 2022(4), M1484. https://doi.org/10.3390/M1484






