Abstract
The title compound, obtained by base-induced dimerization of 3-benzyloxazolidin-2-one, has been prepared and fully characterised. Its X-ray structure features hydrogen-bonded dimers involving the hydroxyl OH and amide carbonyl, forming a 14-membered ring.
1. Introduction
We recently described the [1,2] -Wittig rearrangement of ortho-, meta- and para-benzyloxy-N-butylbenzamides as a convenient synthetic route to substituted diarylmethanols [1] and the corresponding reaction of substituted 2-(2-benzyloxyphenyl)oxazolines is also successful in some cases [2]. However, despite recent advances in various aspects of this reaction [3], its extension to benzyloxy-substituted heterocycles has only been achieved in a few cases. Dudley and coworkers reported Wittig rearrangement of 2-benzyloxypyridines [4] and related systems such as benzyloxy-quinolines and -isoquinolines [5], and we have described the rearrangement of a few appropriately substituted benzyloxythiophenes [6] although competing processes may intervene in some cases.
In an attempt to extend the rearrangement to 2-(benzyloxy)oxazoles, we planned to access 2-benzyloxyoxazoline 2 by O-alkylation of oxazolidine-2-one 1 (Scheme 1) but found that this in fact results in formation of the isomeric N-benzyl product 3. Since there was initially some doubt as to whether 2 or 3 had been obtained, we proceeded with base treatment anyway, and obtained a new crystalline compound 4 which turned out to be the title compound, clearly formed by attack of the anion derived from 3 on a second molecule of 3 with ring opening. We describe here the formation and characterisation of 4 as well as its X-ray structure.
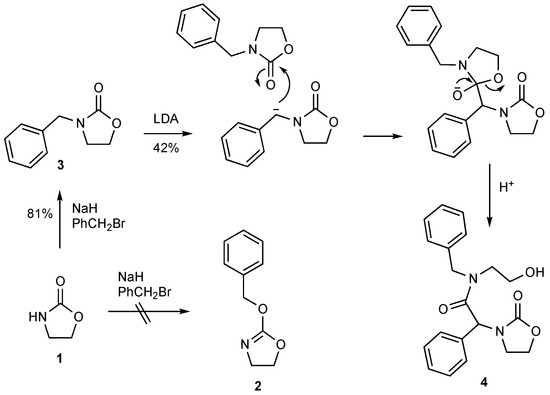
Scheme 1.
Formation and structure of the title compound 4.
2. Results
Alkylation of the commercially available oxazolidinone 1 with sodium hydride and benzyl bromide in THF gave a crystalline product in 81% yield, which proved not to be the target 2-(benzyloxy)oxazoline 2 but rather the isomeric 3-benzyloxazolidin-2-one 3, giving melting point and 1H and 13C NMR data in good agreement with literature values [7]. When this was treated with LDA at −78 °C for 15 min followed by warming to RT over 1 h, aqueous work-up followed by column chromatography gave a crystalline product shown by mass spectrometry to be a dimer of 3. The IR spectrum (Supplementary Material) showed an OH signal at 3454 cm−1 and a new C=O stretch signal at 1638 cm−1 in the range for acyclic amides, in addition to one at 1736 cm−1 consistent with the presence of an oxazolidinone. The NMR spectra were unexpectedly complex and were consistent with the presence of two closely similar isomers in a 10:9 ratio. A distinctive CH signal (δH 6.20/5.96, δC 57.9/58.4) was correlated by HMBC to both amide (δC 170.5/171.6) and oxazolidinone (δC 159.0/158.5) carbonyl carbons. The data were thus consistent with structure 4, formed as shown by nucleophilic attack of the anion of 3 on a second molecule of 3 with subsequent ring-opening (Scheme 1).
The complexity of the NMR data is attributed to the presence of amide rotamers in solution and the slight predominance of one of these allowed unambiguous assignment of many but not all of the signals to major and minor isomers (see Experimental Section).
Since the structure of 4 seemed to offer interesting opportunities for hydrogen bonding and to confirm it unambiguously, a single crystal X-ray diffraction study was carried out. The resulting molecular structure is shown in Figure 1.

Figure 1.
Two alternative views of the molecular structure of 4 (50% probability ellipsoids).
The crystal structure was found to consist of hydrogen bonded dimers (Figure 2, Table 1) with the bonding between the amide carbonyl of one molecule and the primary alcohol OH of the other, thus forming a 14-membered ring: in terms of the Etter–Bernstein [8] graph set description, an R22(14) interaction.
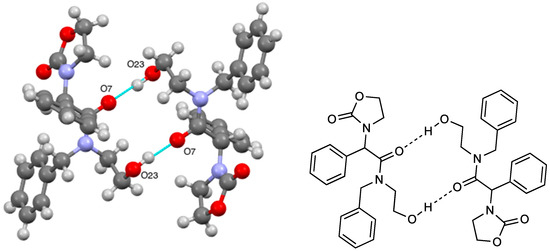
Figure 2.
Hydrogen bonding pattern for compound 4 together with schematic representation.

Table 1.
Hydrogen bonding parameters for 4 (Å, °).
In summary, the title compound has been prepared by base-induced self-condensation of N-benzyloxazolidin-2-one and is shown to exist in solution as an almost 1:1 ratio of amide rotamers while the X-ray crystal structure features head-to-tail dimers formed by hydrogen bonding between the amide carbonyl and the alcohol OH and involving a 14-membered ring.
3. Experimental
Melting points were recorded on a Reichert hot-stage microscope (Reichert, Vienna, Austria) and are uncorrected. IR spectra were recorded on a Perkin-Elmer 1420 instrument (Perkin-Elmer, Waltham, MA, USA). NMR spectra were obtained for 1H at 400 MHz and for 13C at 100 MHz using a Bruker AV II 400 instrument (Bruker, Billerica, MA, USA). Spectra were run at 25 °C on solutions in CDCl3 with internal Me4Si as the reference. Chemical shifts are reported in ppm to high frequency of the reference and coupling constants J are in Hz. N-Benzyloxazolidinone 3 was prepared using a literature method [7] by reaction of commercially available oxazolidinone 1 with NaH in THF followed by BnBr (81% yield)
N-Benzyl-N-(2-hydroxyethyl)-2-(oxazolidin-2-on-3-yl)-2-phenylacetamide (4)
A solution of LDA in THF was generated in situ using BunLi in hexanes (1.58 mL, 3.10 mmol) and Pri2NH (0.44 mL, 3.10 mmol) under a nitrogen atmosphere. A solution of compound 3 (0.53 g, 2.99 mmol) in THF (10 mL) was added and the reaction stirred for 15 min at −78 °C and a further hour at room temperature. The mixture was quenched by addition to saturated aq. NH4Cl (20 mL) and the mixture was extracted with Et2O (3 × 10 mL). Drying and evaporation of the organic extract followed by column chromatography (SiO2, 5:1 EtOAc/hexane) gave 4 (0.22 g, 42%) as a colourless powder, mp 119–122 °C. IR (ATR) 3455 (OH), 1736 (C=O), 1638 (C=O), 1420, 1221, 1084, 1067, 862, 754 cm−1; 1H NMR (400 MHz, CDCl3) [‡ = major amide rotamer, * = minor amide rotamer] 7.45–7.12 (20H, m, Ar-H), 6.20 (1H, s, CH)‡, 5.96 (1H, s, CH)*, 5.09 (1H, d, J 15.0, CH2-O)*, 4.55 (1H, d, J 16.5, CH2-O)‡, 4.38 (2H, m, CH2), 4.33 (1H, d, J 15.0, CH2-O)*, 4.26 (1H, d, J 16.5, CH2-O)‡, 4.20 (2H, m, CH2)‡, 4.15 (2H, m, CH2), 3.84 (1H, m, CH2-N)‡, 3.80 (1H, m, CH2)*, 3.75 (2H, m, CH2)‡, 3.63 (1H, m, CH2)*, 3.50 (1H, m, CH2-N)*, 3.25 (1H, m, CH2-N)‡, 3.08 (1H, m, CH2-N)*, 3.04 (2H, m, CH2)*. 13C NMR (100 MHz, CDCl3) 171.6 (CO)*, 170.5 (CO)‡, 159.0 (CO)‡, 158.5 (CO)*, 137.0 (C)‡, 135.1 (C)*, 133.2 (C)*, 132.9 (C)‡, 129.4 (2CH), 129.2 (2CH), 129.1 (2CH), 129.0 (2CH), 128.9 (2CH), 128.64 (2CH), 128.60 (2CH), 127.9 (CH), 127.8 (2CH), 127.4 (CH), 127.0 (2CH), 63.1 (CH2)‡, 62.8 (CH2)*, 61.3 (CH2)*, 60.2 (CH2)‡, 58.4 (CH)‡, 57.9 (CH)*, 52.1 (CH2)*, 49.3 (CH2)‡, 49.0 (CH2)*, 48.9 (CH2)*, 43.2 (CH2)*, 42.8 (CH2)‡. HRMS (ESI): found 377.1466. calcd for C20H22N2NaO4 (M + Na) 377.1477.
Crystal data for C20H22N2O4, M = 354.40 g mol−1, colourless prism, crystal dimensions 0.15 × 0.10 × 0.03 mm, monoclinic, space group P21/c (No. 14), a = 9.4616(9), b = 16.3487(16), c = 11.9391(11) Å, β = 104.073(2)°, V = 1791.4(3) Å3, Z = 4, Dcalc = 1.314 g cm−3, T = 173 K, R1 = 0.0318, Rw2 = 0.0898 for 2930 reflections with I > 2σ(I), and 239 variables. Data were collected using graphite monochromated Mo Kα radiation λ = 0.71073 Å and have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2194099 The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/getstructures. The structure was solved by direct methods and refined by full-matrix least-squares against F2 (SHELXL, Version 2018/3 [9]).
Supplementary Materials
The following is available online, IR, 1H 13C, HSQC and HMBC NMR and HRMS data and cif and check-cif files for 4.
Author Contributions
J.S.L. prepared the compound; A.M.Z.S. collected the X-ray data and solved the structure; R.A.A. designed the study, analysed the data, and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Crystallographic data have been deposited at CCDC and other data is available in the Supporting Materials as noted above.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aitken, R.A.; Harper, A.D.; Inwood, R.A.; Slawin, A.M.Z. Access to diarylmethanols by Wittig rearrangement of ortho-, meta- and para-benzyloxy-N-butylbenzamides. J. Org. Chem. 2022, 87, 4692–4701. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.A.; Harper, A.D.; Inwood, R.A. Further studies on the [1,2]-Wittig rearrangement of 2-(2-benzyloxy)aryloxazolines. Molecules 2022, 27, 3186. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.; Zhang, Y.; Yang, J. The [1,2]- and [1,4]-Wittig rearrangement. Tetrahedron 2020, 76, 130857. [Google Scholar] [CrossRef]
- Yang, J.; Dudley, G.B. [1,2]-Anionic rearrangement of 2-benzyloxypyridine and related pyridyl ethers. J. Org. Chem. 2009, 74, 7998–8000. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wangweerawong, A.; Dudley, G.B. [1,2]-Wittig rearrangement of aromatic heterocycles. Heterocycles 2012, 85, 1603–1606. [Google Scholar] [CrossRef]
- Aitken, R.A.; Harper, A.D.; Slawin, A.M.Z. Rationalisation of patterns of competing reactivity by X-ray structure determination: Reaction of isomeric (benzyloxythienyl)oxazolines with a base. Molecules 2021, 26, 7690. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, G.S.; Owens, D.A.; Dolan, P.L.; Woo, E. The use of 2-oxazolidinones as latent aziridine equivalents 2. Aminoethylation of aromatic amines, phenols and thiophenols. J. Org. Chem. 1992, 57, 6257–6265. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).