4,7-Bis(2,3,3a,8b-tetrahydrocyclopenta[b]indol-4(1H)-yl)-[1,2,5]thiadiazolo[3,4-c]pyridine
Abstract
:1. Introduction
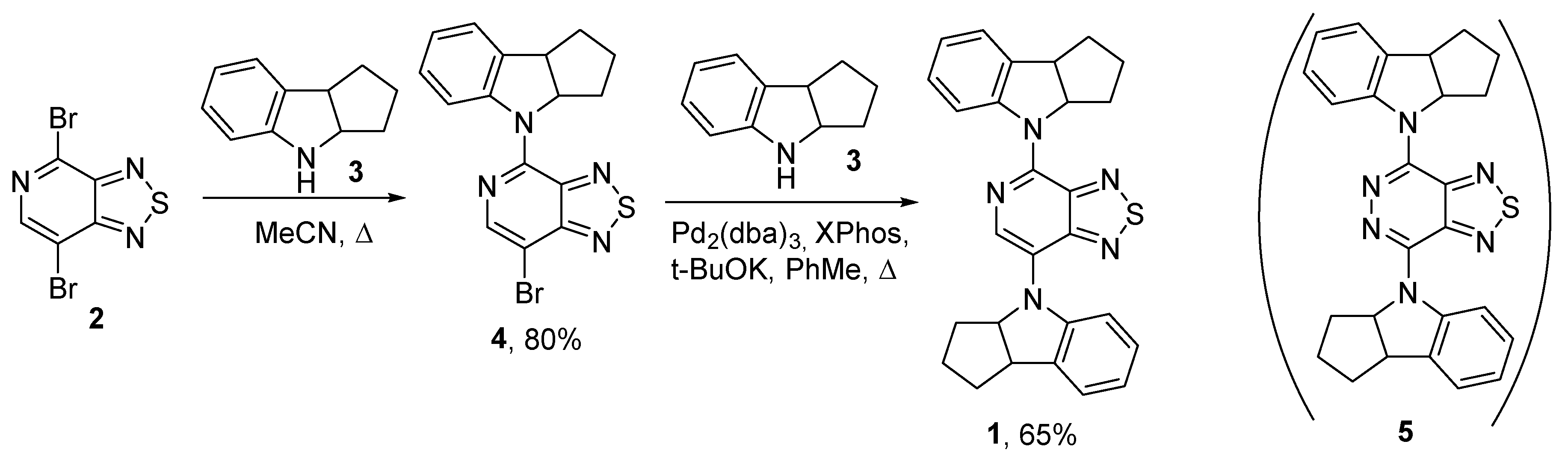
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Wu, G.; He, C.; Deng, D.; Li, Y. Triphenylamine-containing D-A-D molecules with (dicyanomethylene)pyran as an acceptor unit for bulk-heterojunction organic solar cells. J. Mater. Chem. 2011, 21, 3768–3774. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Zhao, R.; Min, Y.; Dou, C.; Lin, B.; Ma, W.; Liu, J.; Wang, L. A Conjugated Polymer Containing a B ← N Unit for Unipolar n-Type Organic Field-Effect Transistors. ACS Appl. Polym. Mater. 2020, 2, 19–25. [Google Scholar] [CrossRef]
- Leventis, A.; Chmovzh, T.N.; Knyazeva, E.A.; Han, Y.; Heeney, M.; Rakitin, O.A.; Bronstein, H. A Novel Low-Bandgap Pyridazine Thiadiazole-Based Conjugated Polymer with Deep Molecular Orbital Levels. Polym. Chem. 2020, 11, 581–585. [Google Scholar] [CrossRef]
- Qian, G.; Dai, B.; Luo, M.; Yu, D.; Zhan, J.; Zhang, Z.; Ma, D.; Wang, Z.Y. Band gap tunable, donor-acceptor-donor charge-transfer heteroquinoid-based chromophores: Near infrared photoluminescence and electroluminescence. Chem. Mater. 2008, 20, 6208–6216. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Chmovzh, T.N.; Golovanov, I.S.; Knyazeva, E.A.; Mikhalchenko, L.V.; Saifutyarov, R.S.; Avetisov, I.C.; Woollins, J.D.; Taydakov, I.V.; Rakitin, O.A. Candle light-style OLEDs with benzochalcogenadiazoles cores. Dyes Pigments 2021, 185, 108917. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Chmovzh, T.N.; Chkhetiani, G.R.; Taydakov, I.V.; Rakitin, O.A. New D–A–D Luminophores of the [1,2,5]Thiadiazolo[3,4-d]Pyridazine Series. Mendeleev Commun. 2022, 32, 371–373. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Rakitin, O.A. Benzobischalcogenadiazoles: Synthesis and applications (microreview). Chem. Heterocycl. Compd. 2022, 58, 307–309. [Google Scholar] [CrossRef]
- Bianchi, L.; Zhang, X.; Chen, Z.; Chen, P.; Zhou, X.; Tang, Y.; Liu, B.; Guo, X.; Facchetti, A. New Benzo[1,2-D:4,5-d′]Bis([1,2,3]Thiadiazole) (Iso-BBT)-Based Polymers for Application in Transistors and Solar Cells. Chem. Mater. 2019, 31, 6519–6529. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Kudryashev, T.A.; Rakitin, O.A. 4,7-Bis(Dodecylthio)-[1,2,5]Thiadiazolo[3,4-c]Pyridine. Molbank 2021, 2021, M1291. [Google Scholar] [CrossRef]
- Mochizuki, M.; Kori, M.; Kono, M.; Yano, T.; Sako, Y.; Tanaka, M.; Kanzaki, N.; Gyorkos, A.C.; Corrette, C.P.; Aso, K. Discovery of a 7-arylaminobenzimidazole series as novel CRF1 receptor antagonists. Bioorg. Med. Chem. 2016, 24, 4675–4691. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chien, S.-C.; Yip, H.-L.; Zhang, Y.; Chen, K.-S.; Zeigler, D.F.; Chen, F.-C.; Lin, B.; Jen, A.K.-Y. High-mobility low-bandgap conjugated copolymers based on indacenodithiophene and thiadiazolo[3,4-c]pyridine units for thin film transistor and photovoltaic applications. J. Mater. Chem. 2011, 21, 13247–13255. [Google Scholar] [CrossRef]
- Scott, T.L.; Burke, N.; Carrero-Martínez, G.; Söderberg, B.C.G. Synthesis of 1,2,3,4-tetrahydrocarbazoles and related tricyclic indoles. Tetrahedron 2007, 63, 1183–1190. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Rakitin, O.A. 4,7-Bis(2,3,3a,8b-tetrahydrocyclopenta[b]indol-4(1H)-yl)-[1,2,5]thiadiazolo[3,4-c]pyridine. Molbank 2022, 2022, M1441. https://doi.org/10.3390/M1441
Chmovzh TN, Rakitin OA. 4,7-Bis(2,3,3a,8b-tetrahydrocyclopenta[b]indol-4(1H)-yl)-[1,2,5]thiadiazolo[3,4-c]pyridine. Molbank. 2022; 2022(3):M1441. https://doi.org/10.3390/M1441
Chicago/Turabian StyleChmovzh, Timofey N., and Oleg A. Rakitin. 2022. "4,7-Bis(2,3,3a,8b-tetrahydrocyclopenta[b]indol-4(1H)-yl)-[1,2,5]thiadiazolo[3,4-c]pyridine" Molbank 2022, no. 3: M1441. https://doi.org/10.3390/M1441
APA StyleChmovzh, T. N., & Rakitin, O. A. (2022). 4,7-Bis(2,3,3a,8b-tetrahydrocyclopenta[b]indol-4(1H)-yl)-[1,2,5]thiadiazolo[3,4-c]pyridine. Molbank, 2022(3), M1441. https://doi.org/10.3390/M1441






