Abstract
X-ray crystallography was used to characterise the title compound for the first time, and the 1H NMR, 13C NMR and IR spectroscopic data from earlier reports were also updated.
1. Introduction
The [4 + 2] cycloaddition reaction of phencyclone 1 and maleic anhydride 2 in refluxing benzene [1] or toluene [2] affords cycloadduct 3 as the sole product (Scheme 1).

Scheme 1.
Diels–Alder reaction of phencyclone (1) with maleic anhydride (2) to form cycloadduct 3.
Cycloadduct 3 was used in studies on the control of reactivity and regioselectivity of cycloaddition reactions of phencyclone with a range of electron-deficient dieneophiles [1]. Compound 3 has also featured in studies of photochemical decarbonylation reactions of norbornene-7-ones [3]. More recently, 3 has been used as an intermediate to prepare maleimides, and the formation of compound 5 is shown as an example in Scheme 2 [2]. Compound 5 is an example of a “molecular balance”: this type of molecule allows interactions between alkyl C—H bonds and aryl π-systems to be investigated [2,4,5,6]. Maleimides such as 5 (Figure 1) exhibit restricted rotation around the N–C bond, and the resulting “unfolded” (5a) and “folded” (5b) conformers can be probed using 1H NMR spectroscopy.
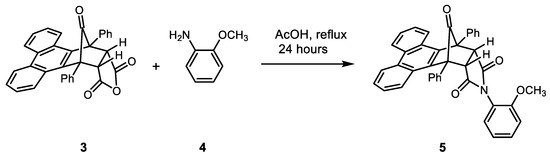
Scheme 2.
Reaction of compound 3 with 2-methoxyaniline (4) to form maleimide 5.

Figure 1.
“Unfolded” and “folded” conformers of maleimide 5 (the CH–π interaction is indicated on 5b as a dashed line).
2. Results
Phencyclone 1 readily reacts with maleic anhydride 2 in refluxing benzene or toluene, however, toluene was preferred for use in this work. The reaction is usually complete within 20–30 min, the end point is readily identified since phencyclone forms a dark green solution in toluene, which fades as the starting material is consumed. Phencyclone can be purchased from commercial suppliers, alternatively, it can easily be prepared from 1,3-diphenylacetone and 9,10-phenanthraquinone [7,8]. Cycloadduct 3 can be stored in a cool, dark place for several years without significant decomposition, however, it is not stable if heated to melting point. Under these conditions, decomposition will occur, and the vigorous release of CO gas will take place as soon as the melting point is reached [1]. The stability of 3 is also limited if stored in hygroscopic solvents, on prolonged storage, the hydrolysis of the cyclic anhydride can take place.
Material that was satisfactory for study by X-ray crystallography was obtained by dissolving samples of compound 3 (0.05–0.1 g) in ethyl acetate (5–10 mL). Where necessary, the mixtures were heated to ensure that all the solid material had dissolved. The samples were stored for several days, until suitable crystals were formed and isolated. The crystal structure of 3 (Figure 2) confirms the results from earlier studies that the compound is isolated as the endo-isomer [1,2]. As noted above, cycloadduct 3 releases carbon monoxide gas when heated to the melting point, and this phenomenon is known to occur in other phencyclone cycloadducts [8,9] and it is attributed to a cheletropic CO extrusion reaction. In a published study, crystallography techniques were used to survey certain structural characteristics of bridged cyclopentenones and comparisons were made to their reactivity in cheletropic decarbonylation reactions [9]. It was concluded that the C=O and highlighted C–C bond distances in bridged cyclopentenones can be useful indicators of the potential for cheletropic decarbonylation to take place. The survey of bridged cyclopentenone structures indicated that C-C bonds broken during the cheletropic reaction are typically longer than those expected for the associated cyclopentanone. In contrast, the C=O bond (that can be lost as CO) is shorter than would be expected in the corresponding bridged cyclopentanone [9]. The crystal structure of 3 has allowed the relevant bond distances to be measured and compared with those expected. In this case, the C1–O1 bond distance is 1.199(4) Å, the C2–C3 bond distance is 1.539(4) Å and the C16–C17 bond distance is 1.535(4) Å. These data are consistent with similar structures reported in the literature [8,9], for comparison, the relevant C–C and C=O bond distances in bridged cyclopentanones are typically 1.519 Å and 1.207 Å, respectively [9].

Figure 2.
Crystal structure of 3 with key atoms highlighted and hydrogen atoms omitted.
The IR spectrum of 3 would be expected to show a high-frequency C=O stretch due to bond angle compression in the cyclopentenone ring [10] (the crystal structure shows that the C2–C1–C17 bond angle is 99.4(2)°). There is an absorption at 1792 cm−1 which is consistent with this expectation (an IR spectrum has been provided in the Supporting Information document), however, the anhydride C=O (asymmetric, out of phase stretch) signal also likely occurs in the same region of the IR spectrum [11]. In this case, both C=O signals are likely to be very close together and may not be resolved. The anhydride C=O (symmetric, in-phase stretch) signal is less intense but is visible at 1855 cm−1. The 13C NMR spectrum of compound 3 confirms the presence of the ketone and anhydride carbonyl groups (195.0 and 170.6 ppm, respectively, are present).
The NMR spectra indicate that the bridgehead phenyl groups exhibit restricted rotation around the C(sp2)–C(sp3) bond (1H and 13C NMR spectra have been provided in the Supporting Information document). Rapid C–C rotation on the NMR timescale would be expected to lead to the observation of three chemical shift environments associated with the phenyl groups. In this example, five signals in the 1H NMR spectrum can be attributed to the phenyl ring protons. This phenomenon has previously been noted in the spectra of [4 + 2] cycloadducts obtained from phencyclone and maleimides and detailed spectroscopic studies of these compounds have been reported [12]. The restricted rotation of phenyl groups in this class of compounds was ascribed to steric interactions between the ortho-protons of the phenyl ring and protons in close proximity from the adjacent phenanthrene ring system (Figure 3).

Figure 3.
Illustration of the protons associated with steric interactions that result in the restricted rotation of bridgehead phenyl group.
In summary, the first X-ray crystal structure of 3 was obtained which confirms the findings of previous studies that endo-cycloadduct 3 is the product from the [4 + 2] cycloaddition reaction of phencyclone 1 with maleic anhydride 2. The crystallographic data provides useful insight into certain spectroscopic properties and thermal decomposition behaviour of the title compound.
3. Experimental Section
Melting points were recorded on an SMP3 melting point apparatus and are uncorrected. IR spectra were recorded on a Perkin Elmer Spectrum Two instrument with DTGS detector and diamond ATR attachment. NMR spectra were obtained for 1H at 500 MHz and for 13C at 125 MHz using a Bruker AVIII 500 instrument. Spectra were run at 25 °C in CD3SOCD3. Chemical shifts are reported in ppm to high frequency of the reference and coupling constants J are reported in Hz.
(9R,9aS,12aR,13S)-9,13-Diphenyl-9,9a,12a,13-tetrahydro-9,13-methanotriphenyleno[2,3-c]furan-10,12,14-trione (3)
A solution of phencyclone 1 (0.5 g, 1.3 mmol) and maleic anhydride 2 (0.32 g, 3.25 mmol) in toluene (10 mL) was heated under reflux for 15–20 min (until the green colour due to dissolved phencylone was no longer visible). Upon cooling to room temperature, methanol (5 mL) was added to the reaction flask and the resulting mixture was cooled in an ice bath for 15 min. A colourless solid was formed, which was filtered off and washed with ice-cold methanol (3 × 5 mL) to afford product 3 (0.45 g, 72%) as a colourless solid, mp 298–300 °C (lit. [1] 296–298 °C). IR (ATR) 3035 (ArCH) 1855 (C=O), 1792 (C=O), 1498, 1448, 900, 770, 758, 695, 508 cm−1; 1H NMR (500 MHz, CD3SOCD3); 8.93 (2H, d, J = 8.4 Hz, ArH), 8.18 (2H, d, J = 7.8 Hz, PhH), 7.82 (2H, apparent t, J = 7.7 Hz, PhH), 7.71–7.55 (4H, m, overlapping ArH and PhH), 7.50 (2H, apparent t, J = 7.5 Hz, PhH), 7.30 (2H, apparent t, J = 7.8 Hz, ArH), 7.23 (2H, d, J = 7.8 Hz, PhH), 7.06 (2H, d, J = 8.4 Hz, ArH), 5.17 (2H, s, CH); 13C NMR (125 MHz, CD3SOCD3) 195.0 (C=O), 170.6 (C=O), 133.9 (ArCq), 133.7 (ArCq), 131.4 (ArCq), 131.4 (ArCH), 129.8 (ArCH), 129.0 (overlapping, 3 × ArCH), 127.9 (ArCH), 127.1 (ArCH), 126.0 (ArCq), 125.4 (ArCH), 124.3 (ArCH), 63.0 (C-Ph), 46.9 (CH).
Colourless X-ray quality crystals of 3 were grown from ethyl acetate solution. X-ray diffraction data for compound 6 were collected at 173 K using a Rigaku FR-X Ultrahigh Brilliance Microfocus RA generator/confocal optics with XtaLAB P200 diffractometer [Mo Kα radiation (λ = 0.71073 Å)]. Intensity data were collected using ω steps accumulating area detector images spanning at least a hemisphere of reciprocal space. Data were collected using CrystalClear [13] and processed (including correction for Lorentz, polarization and absorption) using CrysAlisPro [14]. The structure was solved by dual-space methods (SHELXT) [15] and refined by full-matrix least-squares against F2 (SHELXL-2018/3) [16]. Non-hydrogen atoms were refined anisotropically, and hydrogen atoms were refined using a riding model. All calculations were performed using the Olex2 [17] interface. The showed structure was refined as a two-component, non-merohedral twin, with refined twin fractions of 0.704(5):0.296(5). CCDC 2192702 contains supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
Crystal data for C33H20O4 (M = 480.49): triclinic, space group P (no. 2), a = 9.9060(6), b = 11.1504(7), c = 11.9180(11) Å, α = 117.827(8), β = 94.797(6), γ = 100.060(5)°, V = 1125.47(16) Å3, Z = 2, T = 173 K, μ(Mo Kα) = 0.093 mm−1, ρ (calc) = 1.418 g/cm3, 14793 reflections measured (3.936° ≤ 2θ ≤ 58.010°), 4911 unique (Rint = 0.0472, Rsigma = 0.2), which were used in all calculations. The final R1 [I > 2σ(I)] was 0.0757 and wR2 (all data) was 0.2567.
Supplementary Materials
The following are available online, Figure S1: IR spectrum of 3; Figure S2: 1H NMR spectrum of 3; Figure S3: 13C NMR spectrum of 3; Cif and check-cif files for compound 3.
Author Contributions
This work has been conducted as part of a research project involving honours level undergraduate students (C.B., L.A.C., D.C., T.R.M., E.S.-M.), all of the required synthetic steps, crystallisation trials and preliminary analysis have been conducted by this group; D.B.C. collected the x-ray data and solved the structure; S.R.S. and T.L. acquired and analysed all of the NMR spectroscopy data; I.A.S. and B.A.C. designed the study, analysed the data, and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
CCDC 2192702 contains the supplementary crystallographic data for this paper (Access date 27 July 2022). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
Acknowledgments
This work was conducted as part of an undergraduate group research project at the University of St Andrews. The authors express gratitude to the University of St Andrews School of Chemistry for use of their laboratory facilities and provision of materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sasaki, T.; Kanematsu, K.; Iizuka, K. Molecular design by cycloaddition reactions XXV. High peri- and regiospecificity of phencyclone. J. Org. Chem. 1976, 41, 1105–1112. [Google Scholar] [CrossRef]
- Carroll, W.R.; Zhao, C.; Smith, M.D.; Pellechia, P.J.; Shimizu, K.D. A molecular balance for measuring aliphatic CH- π interactions. Org. Lett. 2011, 13, 4320–4323. [Google Scholar] [CrossRef]
- Fuchs, B. Photochemical behaviour of bridged compounds. Part II. Photolytic and some ground state reactions of carbonyl-bridged anhydrides and their decarbonylation products. J. Chem. Soc. C 1968, 68–71. [Google Scholar] [CrossRef]
- Zhao, C.; Parrish, R.M.; Smith, M.D.; Pellechia, P.J.; Sherrill, C.D.; Shimizu, K.D. Do deuteriums form stronger CH- π interactions? J. Am. Chem. Soc. 2012, 134, 14306–14309. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Li, P.; Carroll, W.R.; Smith, M.D.; Pellechia, P.J.; Shimizu, K.D. Additivity of Substituent Effects in Aromatic Stacking Interactions. J. Am. Chem. Soc. 2014, 136, 14060–14067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, P.; Smith, M.D.; Pellechia, P.J.; Shimizu, K.D. Experimental study of the cooperativity of CH- π interactions. Org. Lett. 2014, 16, 3520–3523. [Google Scholar] [CrossRef]
- Harrison, E.A. The importance of temperature control in the preparation of phencyclone (1,3-diphenyl-2-H-cyclopenta[I]phenanthrene-2-one). J. Chem. Educ. 1992, 69, 571. [Google Scholar] [CrossRef]
- Smellie, I.A.; Carpenter-Warren, C.L.; Chalmers, B.A.; Cordes, D.B.; De A Gouy, R.P.F.; Keddie, N.S.; Lebl, T.; Patterson, I.L.J.; Slawin, A.M.Z. Simple and inexpensive method for the detection of carbon monoxide released from thermal cheletropic decarbonylation reactions . J. Chem. Educ. 2021, 98, 3608–3613. [Google Scholar] [CrossRef]
- Yit Wooi, G.; White, J.M. Structural Manifestations of the Cheletropic Reaction. Org. Biomol. Chem. 2005, 3, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Hoang, G.T.; Kubo, T.; Young, V.G.; Kautzky, J.A.; Wissinger, J.E. Illustrating the utility of X-ray crystallography for structure elucidation through a tandem aldol Condensation/Diels–Alder reaction sequence. J. Chem. Educ. 2015, 92, 1381–1384. [Google Scholar] [CrossRef]
- Dauben, W.G.; Epstein, W.W. Infrared spectra of some cyclic anhydrides. J. Org. Chem. 1959, 24, 1595–1596. [Google Scholar] [CrossRef]
- Callahan, R.; Ramirez, O.; Rosmarion, K.; Rothchild, R.; Bynum, K.C. Multinuclear NMR Studies and ab initio structure calculations of hindered phencyclone Diels-Alder adducts from symmetrical cyclic dienophiles: Cyclohexene, vinylene carbonate, vinylene trithiocarbonate and two N-aryl maleimides. J. Heterocycl. Chem. 2005, 42, 889–898. [Google Scholar] [CrossRef]
- CrystalClear-SM Expert; v2.1; Commercial software; Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2015.
- CrysAlisPro; v1.171.38.46; Commercial software; Rigaku Oxford Diffraction, Rigaku Corporation: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).