2,6-Bis(phenylamino)-4-(iminophenyl)benzoquinone
Abstract
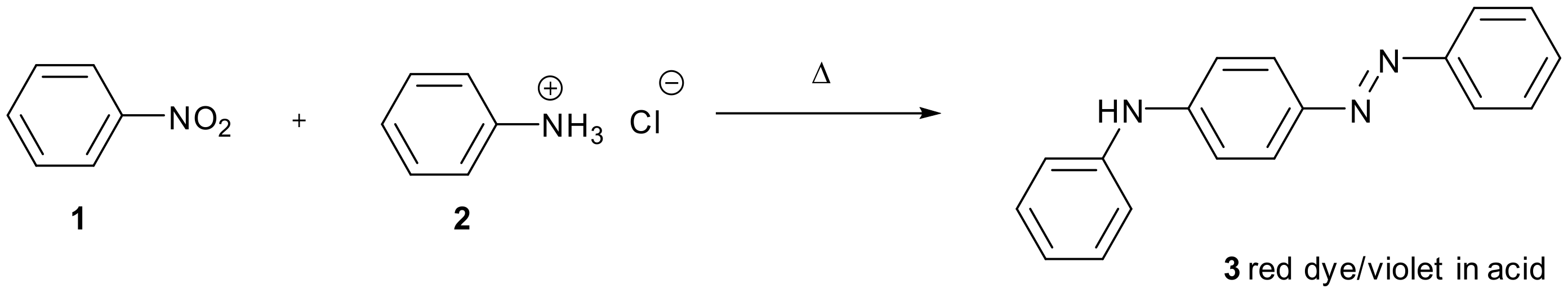
1. Introduction
2. Discussion
3. Materials and Methods
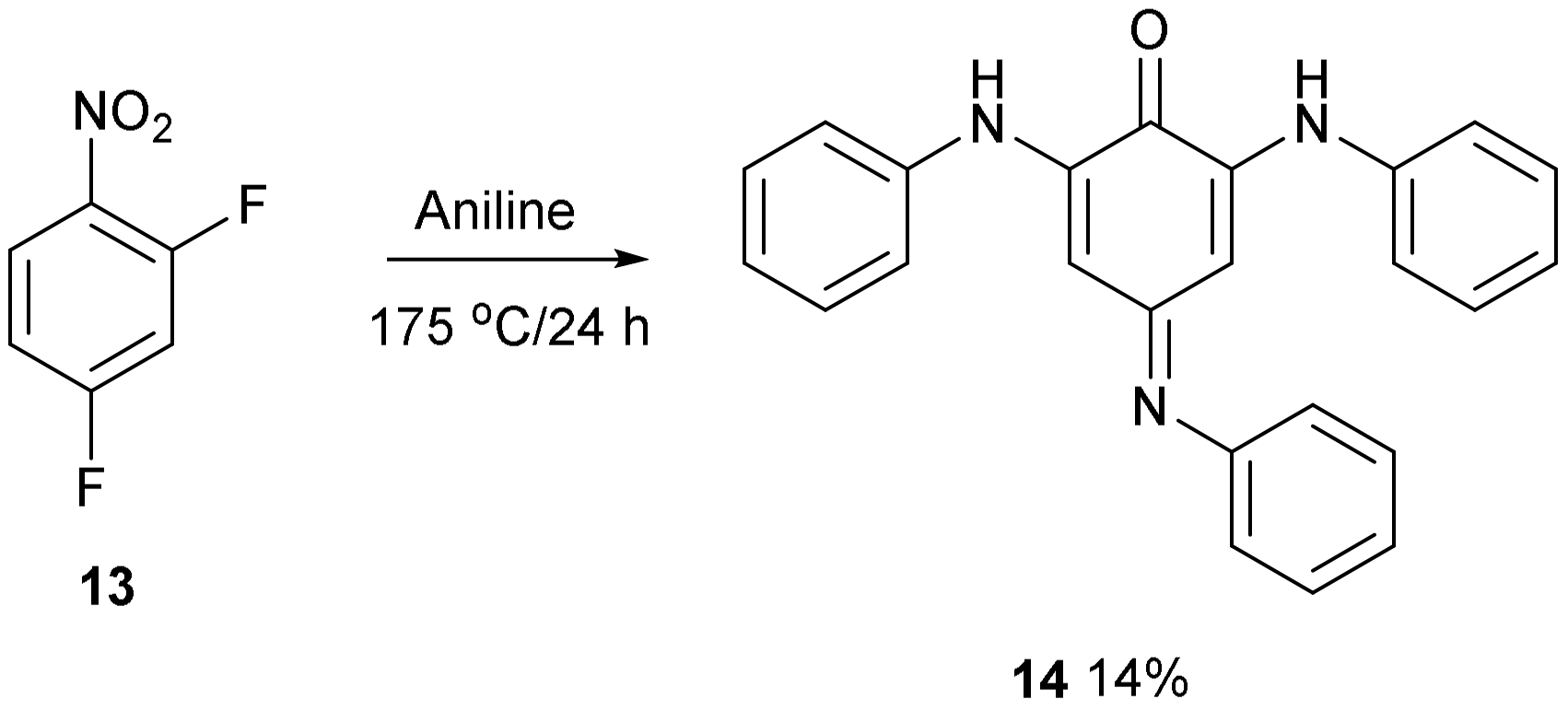
- Synthesis of 2,6-Bis(phenylamino)-4-(iminophenyl)benzoquinone (14)
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Holliday, J. Preparing Colouring Matters. GB Patent 2564, 6 October 1865. [Google Scholar]
- Beska, E.; Toman, P.; Fiedler, K.; Hronec, M.; Pinter, J. Method of Preparation of 4-Aminodiphenylamine. U.S. Patent 6,388,136 B1, 14 May 2002. [Google Scholar]
- Plater, M.J. A synthesis of pseudo-mauveine and a homologue. J. Chem. Res. 2011, 35, 304–309. [Google Scholar] [CrossRef]
- Ohsawa, Y.; Takemura, K.; Seki, A. Photoacid Generators, Chemically Amplified Resist Compositions, and Patterning Process. U.S. Patent 7494760 B2, 24 February 2009. [Google Scholar]
- Ohsawa, Y.; Maeda, K.; Watanabe, S. Photoacid Generators, Chemically Amplified Resist Compositions, and Patterning Process. U.S. Patent 0292768 A1, 20 December 2007. [Google Scholar]
- Plater, M.J.; Williamson, W.T.A.; Raab, A. Photochemical fragmentation of Irgacure PAG 103. ACS Omega 2019, 4, 19875–19879. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Yamato, H.; Tanaka, K.; Takahashi, R.; Kura, H.; Nakano, T. Studies on photodecomposition of an oxime sulfonate. J. Photopolym. Sci. Technol. 2014, 27, 227–230. [Google Scholar] [CrossRef][Green Version]
- Rad, N.I.; Teslenko, Y.O.; Obushak, M.D.; Matiychuk, V.S.; Lytvyn, R.Z.J. Oximes as products in the reactions of 5-substituted 2-nitrothiophenes with arylacetonitriles. J. Heterocycl. Chem. 2011, 48, 1371–1374. [Google Scholar] [CrossRef]
- Chuckowree, I.; Syed, M.A.; Getti, G.; Patel, A.P.; Garner, H.; Tizzard, G.J.; Coles, S.J.; Spencer, J. Synthesis of a 1,3,5-benzotriazepine-2,4-dione based library. Tetrahedron Lett. 2012, 53, 3607–3611. [Google Scholar] [CrossRef]
- Mortzfeld, F.B.; Pietruszka, J.; Baxendale, I.R. A Simple and Efficient Flow Preparation of Pyocyanin a Virulence Factor of Pseudomonas aeruginosa. Eur. J. Org. Chem. 2019, 2019, 5424–5433. [Google Scholar] [CrossRef]
- Lord MFG CO ± (Lord Manufacturing Company). Reacting Oximes with Nitric Oxide and the Products of the Reaction. GB991620A, 12 May 1965. [Google Scholar]
- Plater, M.J.; Harrison, W.T.A. A paddle-wheel motif versus an extended network: Two crystalline forms of 2,4-bis(phenylamino)nitrobenzene. J. Chem. Res. 2015, 39, 98–104. [Google Scholar] [CrossRef]
- Pyne, S.G.; Truscott, R.J.W. Model studies for insect protein sclerotisation: Oxidative loss of the side chain from 4-substituted catechols. Tetrahedron 1990, 46, 661–670. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plater, M.J. 2,6-Bis(phenylamino)-4-(iminophenyl)benzoquinone. Molbank 2022, 2022, M1406. https://doi.org/10.3390/M1406
Plater MJ. 2,6-Bis(phenylamino)-4-(iminophenyl)benzoquinone. Molbank. 2022; 2022(3):M1406. https://doi.org/10.3390/M1406
Chicago/Turabian StylePlater, M. John. 2022. "2,6-Bis(phenylamino)-4-(iminophenyl)benzoquinone" Molbank 2022, no. 3: M1406. https://doi.org/10.3390/M1406
APA StylePlater, M. J. (2022). 2,6-Bis(phenylamino)-4-(iminophenyl)benzoquinone. Molbank, 2022(3), M1406. https://doi.org/10.3390/M1406






