Abstract
The one-pot synthesis methodology has become the leading atom-economical approach for various chemical transformations in a single pot, without purifying intermediate compounds. Chromeno[2,3-b]pyridines are an important class of heterocyclic compounds with versatile biological profiles and other different applications The 5-aminopyrazole system represents a significant heterocyclic template that has gained considerable interest because of its long history of use in the pharmaceutical and agrochemical industries. In this communication, the one-pot transformation of salicylaldehyde, malononitrile dimer, and 2-cyanoacetohydrazide in an ethanol/pyridine mixture was investigated to provide 2,4-diamino-5-(5-amino-3-oxo-2,3-dihydro-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile in good yield. The structure of the novel compound was confirmed by means of elemental analysis, mass-, nuclear magnetic resonance, and infrared spectroscopy.
1. Introduction
The one-pot synthesis methodology has become the leading atom-economical approach for various chemical transformations in a single pot, without purifying intermediate compounds [1]. It avoids the separation process at each step, thus reducing the time of reaction, saving chemical resources, and ensuring a high atom economy. In addition, the process allows you to separate in time and space the reagents that can enter into unwanted reactions and form by-products. It has also been regarded as green chemistry or an eco-friendly approach.
Chromeno[2,3-b]pyridines are an important class of heterocyclic compounds with versatile biological profiles and other different applications. These compounds are a “privileged medicinal scaffold” [2] and have a wide spectrum of biological activity, as well as physicochemical properties that allow them to be used in various industries (for example, as corrosion inhibitors for mild steel [3]). Different substituted chromeno[2,3-b]pyridines are able to exhibit such types of pharmacological activity as antibacterial [4], anticancer [5], antiasthmatic [6], etc. At present, chromenotacrine CT6 (Figure 1) is a non-toxic antioxidant, possessing neuroprotective potent selective inhibitory activity against acetylcholinesterase, which makes it promising for use in the treatment of Alzheimer’s disease [7].
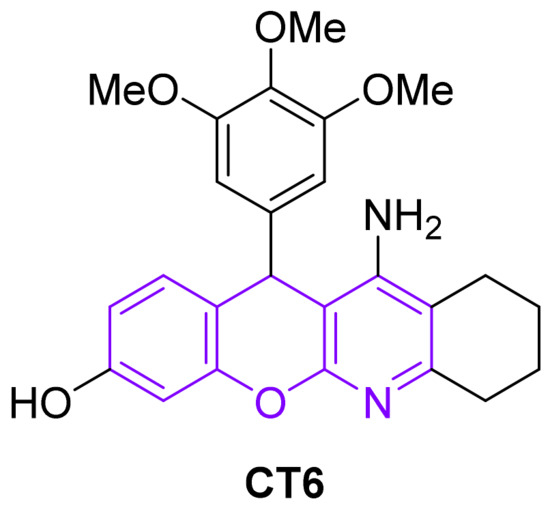
Figure 1.
Chromenotacrine CT6.
The 5-aminopyrazole system represents an important heterocyclic template that has attracted considerable interest because of its long history of application in the pharmaceutical and agrochemical industries [8,9]. 5-Aminopyrazole derivatives are potent gamma-aminobutyric acid (GABA) inhibitors with selectivity toward insect versus mammalian receptors [10], antifungal [11], and antibacterial [12] agents (Figure 2).
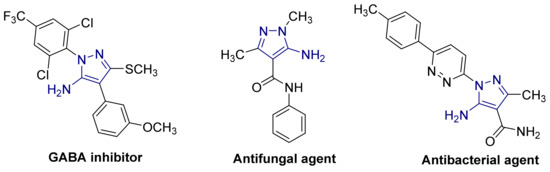
Figure 2.
Some biologically active 5-aminopyrazole derivatives.
Pyrazole derivatives do not exist in nature, probably due to the difficulty in the construction of N–N bonds by living organisms; thus, their availability depends on synthetic methods [13].
In view of significant interest in the synthesis of chromeno[2,3-b]pyridines and 5-aminopyrazoles and their applications, the combination of these fragments in one structure seems promising.
2. Results and Discussion
We have already published a number of studies describing multicomponent reactions leading to substituted chromeno[2,3-b]pyridines [14,15,16,17,18,19].
Now, we wish to report our results on the efficient synthesis of the previously unknown 2,4-diamino-5-(5-amino-3-oxo-2,3-dihydro-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]-pyridine-3-carbonitrile 4.
First, we sought to carry out the transformation of salicylaldehyde 1, 2-aminoprop-1-ene-1,1,3-tricarbonitrile (malononitrile dimer) 2 and 2-cyanoacetohydrazide 3 in a multicomponent variant, as shown in Scheme 1.
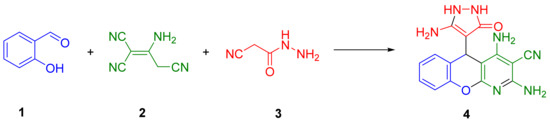
Scheme 1.
Reaction of salicylaldehyde 1, malononitrile dimer 2, and 2-cyanoacetohydrazide 3.
We performed the reaction described above in various systems developed by us [15,16,17,18,20,21]; however, we either obtained a very low yield of the target compound 4 (up to 10%) and a large number of impurities on 1H-NMR spectra or did not observe its formation at all. Based on NMR spectra and the data reported in the literature [22,23,24], we assumed the side process shown in Scheme 2.
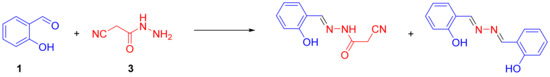
Scheme 2.
Side reaction between starting compounds 1 and 3.
That is why we decided to carry out the reaction in a one-pot format in order to exclude the possibility of a side process (Scheme 3).
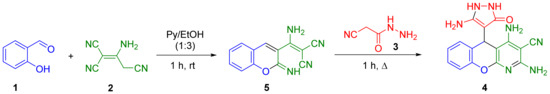
Scheme 3.
One-pot reaction of salicylaldehyde 1, malononitrile dimer 2, and 2-cyanoacetohydrazide 3.
Previously, we studied the first stage of transformation in two catalyst/solvent systems, such as morpholine/acetonitrile and pyridine/ethanol [16,17]. When these reactions were reproduced for 1 h, the yields of compound 5 were comparable (85 and 88%, respectively). However, the second stage proceeded much more efficiently in a mixture of pyridine/ethanol (1:3) (the yield of compound 4 was 83%, while in the morpholine/acetonitrile system it was only 61%). When carrying out the second stage without heating, the yield of chromeno[2,3-b]pyridine 4 decreased to 49%.
Due to the fact that the first stage of the process is subject to kinetic control, we carried it out without heating. The second stage of the process is subject to thermodynamic control, so we carried it out at reflux.
The BFI (bond-forming index) of the process was five since four new bonds were formed in a one-pot reaction—namely, 2 C–C, 2 C–N, and 1 C–O bonds.
The structure of novel 2,4-diamino-5-(5-amino-3-oxo-2,3-dihydro-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4 was confirmed by 1H, 13C-NMR, and IR spectroscopy data, as well as mass spectrometry data and elemental analysis (see Supplementary Materials). Only one set of signals was recorded in 1H and 13C-NMR spectra.
Taking our previous results into consideration (real-time 1H-NMR monitoring data [17,18]) and the data reported in the literature [24], a mechanism for the one-pot reaction of salicylaldehyde 1, 2-aminoprop-1-ene-1,1,3-tricarbonitrile 2, and 2-cyanoacetohydrazide 3 was suggested, as shown in Scheme 4.
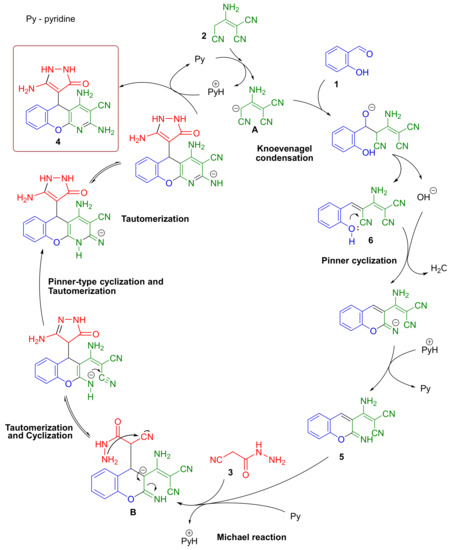
Scheme 4.
Mechanism of one-pot transformation salicylaldehyde 1, malononitrile dimer 2, and 2-cyanoacetohydrazide 3 into chromeno[2,3-b]pyridine 4. Catalytic cycles are simplified.
The first stage of the one-pot process was a rapid formation of Knoevenagel adduct 6 with the expulsion of a hydroxide anion [25]. This hydroxide anion catalyzed a rapid Pinner cyclization of unsaturated adduct 6 into 4-H-chromene intermediate 5. Then, the Michael addition of 2-cyanoacetohydrazide 3 occurred to form anion B. Next, there were successive tautomerizations and simultaneous cyclization to form a 5-aminopyrazolone ring, as well as Pinner-type cyclization to the final 2,4-diamino-5-(5-amino-3-oxo-2,3-dihydro-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4.
3. Materials and Methods
3.1. General Methods
The solvents and reagents were purchased from commercial sources and used as received. 2-Aminoprop-1-ene-1,1,3-tricarbonitrile 2 (malononitrile dimer) was synthesized from malononitrile by the standard dimerization procedure [26]. 2-Cyanoacetohydrazide 4 was obtained from methyl cyanoacetate and hydrazine hydrate according to the literature [27].
The melting point was determined with Gallenkamp melting-point apparatus (Gallenkamp & Co., Ltd., London, UK). 1H and 13C-NMR spectra were registered in DMSO-d6 at ambient temperature. IR spectrum was recorded with a Bruker ALPHA-T FTIR spectrometer (Bruker Corporation, Billerica, MA, USA) in KBr pellets. The MS spectrum (EI = 70 eV) was obtained directly with a Kratos MS-30 spectrometer (Kratos Analytical Ltd., Manchester, UK). For elemental analysis, a 2400 Elemental Analyzer (Perkin Elmer Inc., Waltham, MA, USA) was applied.
3.2. One-Pot Synthesis of 2,4-Diamino-5-(5-amino-3-oxo-2,3-dihydro-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4
Salicylaldehyde 1 (0.122 g, 1 mmol) and 2-aminoprop-1-ene-1,1,3-tricarbonitrile 2 (0.132 g, 1 mmol) were stirred at room temperature in 4 mL of ethanol–pyridine (3:1) mixture for 1 h. A large amount of yellow precipitate (intermediate 5) was observed. Then, 2-cyanoacetohydrazide 3 (1 mmol) was added to the reaction mixture and refluxed for 1 h. The formed solid was filtered, washed with well-chilled ethanol (3 mL × 2), and dried to isolate previously unknown pure 2,4-diamino-5-(5-amino-3-oxo-2,3-dihydro-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4.
2,4-Diamino-5-(5-amino-3-oxo-2,3-dihydro-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4): Yellowish solid; yield—83% (0.278 g); mp > 330 °C (decomp.) (from EtOH); FTIR (KBr) cm−1: 3472 (NH2), 3438 (NH2), 3396 (NH), 3350 (NH2), 3323 (NH2), 3241 (NH2), 3201 (NH2), 2202 (CN), 1637 (C=O), 1600 (C–C Ar), 1602 (C–C Ar), 1585 (C–C Ar), 1568 (C–C Ar). 1H-NMR (300 MHz, DMSO-d6): δ 4.73 (s, 1H, CH), 5.05 (br s, 2H, NH2 pyr), 6.27 (br s, 2H, C(4)-NH2), 6.51 (br s, 2H, C(2)-NH2), 6.96–7.13 (m, 3H, 3 CH Ar), 7.19 (t, 3J = 7.6 Hz, 1H, CH Ar), 9.07 (br s, 2H, 2 NH) ppm; 13C-NMR (75 MHz, DMSO-d6): δ 26.5 (C(5)H), 70.7 (C(3)-CN), 89.6 (C(4’) pyr), 90.9 (C(4a)), 115.9 (C(9)H Ar), 116.5 (CN), 122.9 (C(5a)), 124.0 (C(7)H Ar), 127.8 (C(8)H Ar), 129.3 (C(6)H Ar), 150.3 (C(9a)), 155.4 (C(4)-NH2), 157.1 (C(2)-NH2), 158.5 (C(1a)), 159.1 (C(5’)-NH2 pyr), 169.6 (C(3’)=O) ppm; MS (m/z, relative intensity %): 335 [M]+ (1), 316 [M-H-H2O]+ (3), 237 [M-C3H4N3O]+ (100), 99 [C3H5N3O]+ (33), 41 [C2H3N]+ (88); Anal. calcd. for C16H13N7O2: C, 57.31; H, 3.91; N, 29.24%; found: C, 57.39; H, 3.97; N, 29.18%.
3.3. Synthesis of 2-(Amino(2-imino-2H-chromen-3-yl)methylene)malononitrile 5
Salicylaldehyde 1 (0.122 g, 1 mmol) and 2-aminoprop-1-ene-1,1,3-tricarbonitrile 2 (0.132 g, 1 mmol) were stirred at room temperature in 4 mL of ethanol–pyridine (3:1) mixture for 1 h. After the reaction was finished, the solid was filtered, washed with well-chilled methanol (3 × 2 mL), and dried in vacuum with a water pump to isolate pure 2-(amino-(2-imino-2H-chromen-3-yl)methylene)malononitrile 5.
2-(Amino(2-imino-2H-chromen-3-yl)methylene)malononitrile (5): Yellow solid; yield—80% (0.189 g); mp 270-271 °C (decomp.) (lit (https://doi.org/10.1002/ejoc.201900240) m.p. 271–272 °C (decomp.)); 1H-NMR (300 MHz, DMSO-d6): δ 7.10–7.29 (m, 2H, 2 CH Ar), 7.43–7.61 (m, 2H, 2 CH Ar), 7.75 (s, 1H, CH), 8.66 (s, 1H, NH), 8.93 (br s, 1H, NHH), 8.96 (br s, 1H, NHH) ppm.
4. Conclusions
The title compound, 2,4-diamino-5-(5-amino-3-oxo-2,3-dihydro-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4, was synthesized in good yield using the efficient one-pot method with simple equipment and available starting materials. The process includes Knoevenagel condensation, Pinner reactions, cyclization to form the 5-aminopyrazole cycle, as well as tautomerizations and protonation. The novel compound was characterized using spectroscopic methods (NMR, IR, and MS-EI) and elemental analysis.
Supplementary Materials
The following are available online, compound 4 spectra: 1H-NMR (Figure S1), 13C-NMR (Figure S2), IR (Figure S3), and MS (Figure S4).
Author Contributions
Conceptualization, Y.E.R. and M.N.E.; methodology, Y.E.R. and M.N.E.; validation, Y.E.R. and M.N.E.; investigation, Y.E.R.; resources, M.N.E.; writing—original draft preparation, Y.E.R.; writing—review and editing, M.N.E.; supervision, M.N.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for the compound presented in this study are available in the Supplementary Materials of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hameed, A.; Hameed, A.; Nadeem, H.; Farooq, T. Chapter 5—One-pot synthesis of nanomaterials. In Handbook of Greener Synthesis of Nanomaterials and Compounds; Kharisov, B., Kharissova, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 137–176. [Google Scholar] [CrossRef]
- Barreiro, E.J. Chapter 1. Privileged Scaffolds in Medicinal Chemistry: An Introduction. In Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; Bräse, S., Ed.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 1–15. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Obot, I.B.; Ebenso, E.E.; Quraishi, M.A. 2,4-Diamino-5-(phenylthio)-5H-chromeno[2,3-b]-pyridine-3-carbonitriles as green and effective corrosion inhibitors: Gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv. 2016, 6, 53933–53948. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Tripathi, R.P.; Ramachandran, R. NAD+-dependent DNA Ligase (Rv3014c) from Mycobacterium tuberculosis. Crystal structure of the adenylation domain and identification of novel inhibitors. J. Biol. Chem. 2005, 280, 30273–30281. [Google Scholar] [CrossRef] [Green Version]
- Azuine, M.A.; Tokuda, H.; Takayasu, J.; Enjyo, F.; Mukainaka, T.; Konoshima, T.; Nishino, H.; Kapadia, G.J. Cancer chemopreventive effect of phenothiazines and related tri-heterocyclic analogues in the 12-O-tetradecanoylphorbol-13-acetate promoted Epstein-Barr virus early antigen activation and the mouse skin two-stage carcinogenesis models. Pharmacol. Res. 2004, 49, 161–169. [Google Scholar] [CrossRef]
- Ukawa, K.; Ishiguro, T.; Kuriki, H.; Nohara, A. Synthesis of the metabolites and degradation products of 2-amino-7-isopropyl-5-oxo-5H-(1)benzopyrano(2,3-b)pyridine-3-carboxylic acid (Amoxanox). Chem. Pharm. Bull. 1985, 33, 4432–4437. [Google Scholar] [CrossRef] [Green Version]
- Oset-Gasque, M.J.; González, M.P.; Pérez-Peña, J.; García-Font, N.; Romero, A.; del Pino, J.; Ramos, E.; Hadjipavlou-Litina, D.; Soriano, E.; Chioua, M.; et al. Toxicological and pharmacological evaluation, antioxidant, ADMET and molecular modeling of selected racemic chromenotacrines {11-amino-12-aryl-8,9,10,12-tetrahydro-7H-chromeno[2,3-b]-quinolin-3-ols} for the potential prevention and treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 74, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Elguero, J. 4.04-Pyrazoles and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Charles, W.R., Eds.; Pergamon: Oxford, UK, 1984; Volume 5, pp. 167–303. [Google Scholar] [CrossRef]
- Elguero, J. 3.01—Pyrazoles. In Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Charles, W.R., Scriven, E.F.V., Eds.; Pergamon: Oxford, UK, 1996; Volume 3, pp. 1–75. [Google Scholar] [CrossRef]
- Meegalla, S.K.; Doller, D.; Sha, D.Y.; Soll, R.; Wisnewski, N.; Silver, G.M.; Dhanoa, D. Synthesis and GABA receptor potency of 3-thiomethyl-4-(hetero)aryl-5-amino-1-phenylpyrazoles. Bioorg. Med. Chem. Lett. 2004, 14, 4949–4953. [Google Scholar] [CrossRef]
- Huppatz, J.L. Systemic Fungicides. The Synthesis of Pyrazolo[1,5-a]pyrimidine Analogues of Carboxin. Aust. J. Chem. 1985, 38, 221–230. [Google Scholar] [CrossRef]
- Shamroukh, A.H.; Rashad, A.E.; Sayed, H.H. Synthesis of Some Pyrazolo[3,4]pyrimidine Derivatives for Biological Evaluation. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 2347–2360. [Google Scholar] [CrossRef]
- Aggarwal, R.; Kumar, V.; Kumar, R.; Singh, S.P. Approaches towards the synthesis of 5-aminopyrazoles. Beilstein J. Org. Chem. 2011, 7, 179–197. [Google Scholar] [CrossRef] [Green Version]
- Elinson, M.N.; Ryzhkova, Y.E.; Ryzhkov, F.V. Multicomponent design of chromeno[2,3-b]pyridine systems. Russ. Chem. Rev. 2021, 90, 94–115. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Bushmarinov, I.S.; Zlotin, S.G.; Egorov, M.P. Pot, atom and step economic (PASE) synthesis of 5-isoxazolyl-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun. 2015, 25, 424–426. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. Pot-, Atom- and Step-Economic (PASE) Multicomponent approach to the 5-(Dialkylphosphonate)-Substituted 2,4-Diamino-5H-chromeno-[2,3-b]pyridine scaffold. Eur. J. Org. Chem. 2019, 2019, 4171–4178. [Google Scholar] [CrossRef]
- Ryzhkov, F.V.; Ryzhkova, Y.E.; Elinson, M.N.; Vorobyev, S.V.; Fakhrutdinov, A.N.; Vereshchagin, A.N.; Egorov, M.P. Catalyst-Solvent System for PASE Approach to Hydroxyquinolinone-Substituted Chromeno[2,3-b]pyridines Its Quantum Chemical Study and Investigation of Reaction Mechanism. Molecules 2020, 25, 2573. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Elinson, M.N.; Maslov, O.I.; Fakhrutdinov, A.N. Multicomponent Synthesis of 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonic Acids in DMSO. Molecules 2021, 26, 6839. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Ryzhkov, F.V.; Maslov, O.I.; Elinson, M.N. 2,4-Diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile. Molbank 2022, 2022, M1365. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Novikov, R.A.; Egorov, M.P. Synthesis, structural, spectroscopic and docking studies of new 5C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine-3-carbonitriles. J. Mol. Struct. 2017, 1146, 766–772. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Krymov, S.K.; Fakhrutdinov, A.N.; Egorov, M.P. Potassium fluoride catalysed multicomponent approach to medicinally privileged 5-[3-hydroxy-6-(hydroxymethyl)-4H-pyran-2-yl] substituted chromeno[2,3-b]pyridine scaffold. Arkivoc 2019, 2, 38–49. [Google Scholar] [CrossRef]
- Assiri, M.A.; Al-Sehemi, A.G.; Pannipara, M. AIE based “on-off” fluorescence probe for the detection of Cu2+ ions in aqueous media. Inorg. Chem. Commun. 2019, 99, 11–15. [Google Scholar] [CrossRef]
- Hanna, M.L.; Tarasow, T.M.; Perkins, J. Mechanistic differences between in vitro assays for hydrazone-based small molecule inhibitors of anthrax lethal factor. Bioorg. Chem. 2007, 35, 50–58. [Google Scholar] [CrossRef]
- Mane, M.M.; Pore, D.M. Synthesis of 2-Amino-4-(3-amino-5-hydroxy-4H-pyrazol-4-ylidene)-4H-chromene-3-carbonitriles. Synlett 2016, 27, 1720–1724. [Google Scholar] [CrossRef]
- Patai, S.; Israeli, Y. 411. The kinetics and mechanisms of carbonyl–methylene condensations. Part VII. The reaction of malononitrile with aromatic aldehydes in ethanol. J. Chem. Soc. 1960, 2025–2030. [Google Scholar] [CrossRef]
- Mittelbach, M. An improved and facile synthesis of 2-amino-1,1,3-tricyanopropene. Mon. Chem. Chem. Mon. 1985, 116, 689–691. [Google Scholar] [CrossRef]
- Gorobets, N.Y.; Yousefi, B.H.; Belaj, F.; Kappe, C.O. Rapid microwave-assisted solution phase synthesis of substituted 2-pyridone libraries. Tetrahedron 2004, 60, 8633–8644. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).