4-(Benzo[d]thiazol-2-yl)-1-(2-nitrophenyl)-1H-1,2,3-triazol-5-amine
Abstract
:1. Introduction
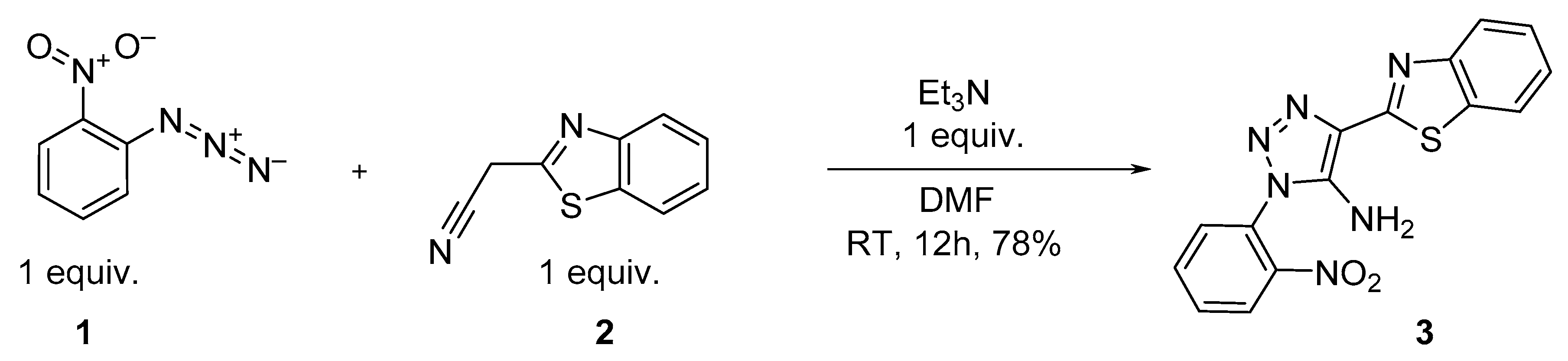
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haddadin, M.J.; Zerdan, R.M.B.; Kurth, M.J.; Fettinger, J.C. Efficient syntheses of the unknown quinolino[2,3-c]cinnolines; synthesis of neocryptolepines. Org. Lett. 2010, 12, 5502–5505. [Google Scholar] [CrossRef]
- Scobie, M.; Tennant, G. A convenient synthesis of otherwise inaccessible 3-aminocinnoline-4-carboxylic acid derivatives. J. Chem. Soc. Chem. Commun. 1994, 1994, 2451–2452. [Google Scholar] [CrossRef]
- D’Alarcao, M.; Bakthavachalam, V.; Leonard, N.J. Synthesis of imidazo[4,5-h]-1,3-diazabiphenylene (lin-benzocyclobutadienopurine), a ring system having a benzocyclobutadieno spacer between the terminal rings of purine. J. Org. Chem. 1985, 50, 2456–2461. [Google Scholar] [CrossRef]
- Haddadin, M.J.; El-Khatib, M.; Shoker, T.A.; Beavers, C.M.; Olmstead, M.M.; Fettinger, J.C.; Farber, K.M.; Kurth, M.J. Quinoxalino[2,3-c]cinnolines and their 5-N-oxide: Alkoxylation of methyl-substituted quinoxalino[2,3-c]cinnolines to acetals and orthoesters. J. Org. Chem. 2011, 76, 8421–8427. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Obushak, M.D. A Convenient Synthesis of [1,2,3]Triazolo[1,5-a]quinoline. Russ. J. Org. Chem. 2019, 55, 1241–1243. [Google Scholar] [CrossRef]
- Shyyka, O.Y.; Pokhodylo, N.T.; Finiuk, N.S. Anticancer activity evaluation of thieno[3,2-e][1,2,3]triazolo[1,5-a] pyrimidines and thieno[2,3-e][1,2,3]triazolo[1,5-a]pyrimidine derivatives. Biopolym. Cell 2019, 35, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Pokhodylo, N.; Shyyka, O.; Finiuk, N.; Stoika, R. Selected 5-amino-1-aryl-1H-1,2,3-triazole scaffolds as promising antiproliferative agents. Ukr. Biochem. J. 2020, 92, 23–32. [Google Scholar] [CrossRef]
- Biagi, G.; Giorgi, I.; Livi, O.; Scartoni, V.; Betti, L.; Giannaccini, G.; Trincavelli, M.L. New 1,2,3-triazolo[1,5-a]quinoxalines: Synthesis and binding to benzodiazepine and adenosine receptors. II. Eur. J. Med. Chem. 2002, 37, 565–571. [Google Scholar] [CrossRef]
- Smalley, R.K.; Teguiche, M. 1,2,3-Triazolo[1,5-a]quinolines, -[1,7]naphthyridines, and -benzo[1,5]diazepines by the action of diethyl 1,3-acetonedicarboxylate anion on ortho-substituted aryl azides. Synthesis 1990, 1990, 654–656. [Google Scholar] [CrossRef]
- Biagi, G.; Giorgi, I.; Livi, O.; Nardi, A.; Scartoni, V. Triazolylbenzimidazolthiones and derivatives of the new 1,2,3-triazolo[1,5-a][1,3,5]benzotriazepine heterocycle. J. Heterocycl. Chem. 2002, 39, 1293–1298. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Shyyka, O.Y.; Obushak, M.D. Convenient synthetic path to ethyl 1-aryl-5-formyl-1H-1,2,3-triazole-4-carboxylates and 1-aryl-1,5-dihydro-4H-[1,2,3]triazolo[4,5-d]pyridazin-4-ones. Chem. Heterocycl. Compd. 2018, 54, 773–779. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Shyyka, O.Y.; Tupychak, M.A.; Obushak, M.D. Selectivity in domino reaction of ortho-carbonyl azides with malononitrile dimer leading to [1,2,3]triazolo[1,5-a] pyrimidines. Chem. Heterocycl. Compd. 2018, 54, 209–212. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Matiychuk, V.S.; Obushak, N.D. Synthesis of 1H-1, 2, 3-triazole derivatives by the cyclization of aryl azides with 2-benzothiazolylacetonone, 1, 3-benzo-thiazol-2-ylacetonitrile, and (4-aryl-1,3-thiazol-2-yl) acetonitriles. Chem. Heterocycl. Compd. 2009, 45, 483–488. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Shyyka, O.Y.; Obushak, M.D. Facile and efficient one-pot procedure for thieno[2,3-e][1,2,3]triazolo[1, 5-a]pyrimidines preparation. Synth. Commun. 2014, 44, 1002–1006. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Matiychuk, V.S.; Obushak, N.D. Synthesis of a new heterocyclic system-pyrido[3′,2′:4,5]thieno-[2,3-e][1,2,3]triazolo[1,5-a]pyrimidine. Chem. Heterocycl. Compd. 2009, 45, 881–883. [Google Scholar] [CrossRef]
- Sharma, P.C.; Sinhmar, A.; Sharma, A.; Rajak, H.; Pathak, D.P. Medicinal significance of benzothiazole scaffold: An insight view. J. Enzyme Inhib. Med. Chem. 2013, 28, 240–266. [Google Scholar] [CrossRef]
- Pokhodylo, N.; Savka, R.; Obushak, M. Comparison of synthetic routes for fully substituted (1H-1,2,3-triazol-4-yl) acetic acids. Curr. Chem. Lett. 2021, 10, 53–66. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Shyyka, O.Y.; Goreshnik, E.A.; Obushak, M.D. 4-Phosphonated or 4-Free 1,2,3-Triazoles: What Controls the Dimroth Reaction of Arylazides with 2-Oxopropylphosphonates? ChemistrySelect 2020, 5, 260–264. [Google Scholar] [CrossRef]
- Shafran, Y.M.; Beryozkina, T.V.; Efimov, I.V.; Bakulev, V.A. Synthesis of β-azolyl-and β-azolylcarbonylenamines and their reactions with aromatic azides. Chem. Heterocycl. Compd. 2019, 55, 704–715. [Google Scholar] [CrossRef]
- Xavier, D.M.; Goldani, B.S.; Seus, N.; Jacob, R.G.; Barcellos, T.; Paixao, M.W.; Luque, R.; Alves, D. Sonochemistry in organocatalytic enamine-azide [3+2]cycloadditions: A rapid alternative for the synthesis of 1,2,3-Triazoyl carboxamides. Ultrason. Sonochem. 2017, 34, 107–114. [Google Scholar] [CrossRef]
- Filimonov, V.O.; Dianova, L.N.; Beryozkina, T.V.; Mazur, D.; Beliaev, N.A.; Volkova, N.N.; Ilkin, V.G.; Dehaen, W.; Lebedev, A.T.; Bakulev, V.A. Water/Alkali-catalyzed reactions of azides with 2-cyanothioacetamides. Eco-friendly synthesis of monocyclic and bicyclic 1,2,3-thiadiazole-4-carbimidamides and 5-amino-1,2,3-triazole-4-carbothioamides. J. Org. Chem. 2019, 84, 13430–13446. [Google Scholar] [CrossRef]
- Krishna, P.M.; Ramachary, D.B.; Peesapati, S. Azide–acetonitrile “click” reaction triggered by Cs2CO3: The atom-economic, high-yielding synthesis of 5-amino-1,2,3-triazoles. RSC Adv. 2015, 5, 62062–62066. [Google Scholar] [CrossRef]
- Belyaev, N.A.; Beryozkina, T.V.; Bakulev, V.A. Synthesis of N-heteroarylamidines of 1,2,3-thiadiazole-4-carboxylic acid from 2-cyanothioacetamides and 5-azido-1-methyl-4-nitroimidazole. Chem. Heterocycl. Compd. 2016, 52, 206–208. [Google Scholar] [CrossRef]
- Tupychak, M.A.; Shyyka, O.Y.; Pokhodylo, N.T.; Obushak, M.D. Nitrileimines as an alternative to azides in base-mediated click [3+2] cycloaddition with methylene active nitriles. RSC Adv. 2020, 10, 13696–13699. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, F.; Aneja, B.; Hasan, P.; Zeya, B.; Zafaryab, M.; Mehdi, S.H.; Rizvi, M.M.A.; Patel, R.; Rana, S.; Abid, M. Synthesis, Anticancer Evaluation and DNA-Binding Spectroscopic Insights of Quinoline-Based 1,3,4-Oxadiazole-1,2,3-triazole Conjugates. ChemistrySelect 2019, 4, 12176–12182. [Google Scholar] [CrossRef]
- Kuleshova, O.; Khilya, O.; Volovenko, Y.; Mallet-Ladeira, S.; Dyakonenko, V.; Gras, E. Expedited route to fully substituted amino-pyrazole building blocks and their further transformations. ACS Omega 2017, 2, 8911–8927. [Google Scholar] [CrossRef]
| Entry | Base | Solvent | Time, h | Triazole 3 Yield, % [a] |
|---|---|---|---|---|
| 1 | MeONa | MeOH | 2 [b] | 41 |
| 2 | Et3N | DMF | 12 | 78 |
| 3 | DBU | DMF | 2 | 5 |
| 4 | K2CO3 | DMSO | 7 | 67 |
| 5 | Cs2CO3 | DMSO | 2 | 53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhodylo, N.T.; Obushak, M.D. 4-(Benzo[d]thiazol-2-yl)-1-(2-nitrophenyl)-1H-1,2,3-triazol-5-amine. Molbank 2022, 2022, M1398. https://doi.org/10.3390/M1398
Pokhodylo NT, Obushak MD. 4-(Benzo[d]thiazol-2-yl)-1-(2-nitrophenyl)-1H-1,2,3-triazol-5-amine. Molbank. 2022; 2022(3):M1398. https://doi.org/10.3390/M1398
Chicago/Turabian StylePokhodylo, Nazariy T., and Mykola D. Obushak. 2022. "4-(Benzo[d]thiazol-2-yl)-1-(2-nitrophenyl)-1H-1,2,3-triazol-5-amine" Molbank 2022, no. 3: M1398. https://doi.org/10.3390/M1398
APA StylePokhodylo, N. T., & Obushak, M. D. (2022). 4-(Benzo[d]thiazol-2-yl)-1-(2-nitrophenyl)-1H-1,2,3-triazol-5-amine. Molbank, 2022(3), M1398. https://doi.org/10.3390/M1398







