Fosbergenone: 3-[2-(1,2,5,5-Tetramethyl-7-oxo-1,2,3,4,4a,5,6,7-octahydronaphthalen-1-yl)ethyl]-2,5-dihydrofuran-2-one
Abstract
:1. Introduction
2. Results
Extraction and Isolation
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Low, Y.W.; Wong, K.M. Two new species of Gardenia (Rubiaceae) from Borneo and notes on Gardenia pterocalyx. Edinb. J. Bot. 2007, 64, 25–36. [Google Scholar] [CrossRef]
- Tirvengadum, D.D. New taxa and name changes in tropical asiatic Rubiaceae. Edinb. J. Bot. 1983, 3, 455–469. [Google Scholar] [CrossRef]
- Herat, T. Endemic Flowering Plants of Sri Lanka; Biodiversity Secretariat of the Ministry of Environment and Natural Resources: Colombo, Sri Lanka, 2007; Part II A; p. 43. [Google Scholar]
- Gunatilaka, A.A.L.; Sirimanne, S.R.; Sotheeswaran, S. Flavonoids of Gardenia cramerii and G. fosbergii bud exudates. Phytochemistry 1982, 21, 805–806. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Ultraviolet spectroscopy. In Introduction to Spectroscopy, 5th ed.; Cengage Learning: Stamford, CT, USA, 2015; p. 596. [Google Scholar]
- Feresin, G.E.; Tapia, A.; Gimenez, A.; Ravelo, A.G.; Zacchino, S.; Sortino, M.; Hirschmann, G.S. Constituents of the Argentinian medicinal plant Baccharis grisebachii and their antimicrobial activity. J. Ethnopharmacol. 2003, 89, 73–80. [Google Scholar] [CrossRef]
- Toyota, M.; Nakamura, I.; Takaoka, S.; Kan, Y.; Asakawa, Y. Terpenoid constituents of the liverwort Heteroscyphus coalitus. Phytochemistry 1996, 41, 575–580. [Google Scholar] [CrossRef]
- Roncero, A.M.; Tobal, I.E.; Moro, R.F.; D’ıez, D.; Marcos, I.S. Halimane diterpenoids: Sources, structures, nomenclature and biological activities. Nat. Prod. Rep. 2018, 35, 955–991. [Google Scholar] [CrossRef] [PubMed]
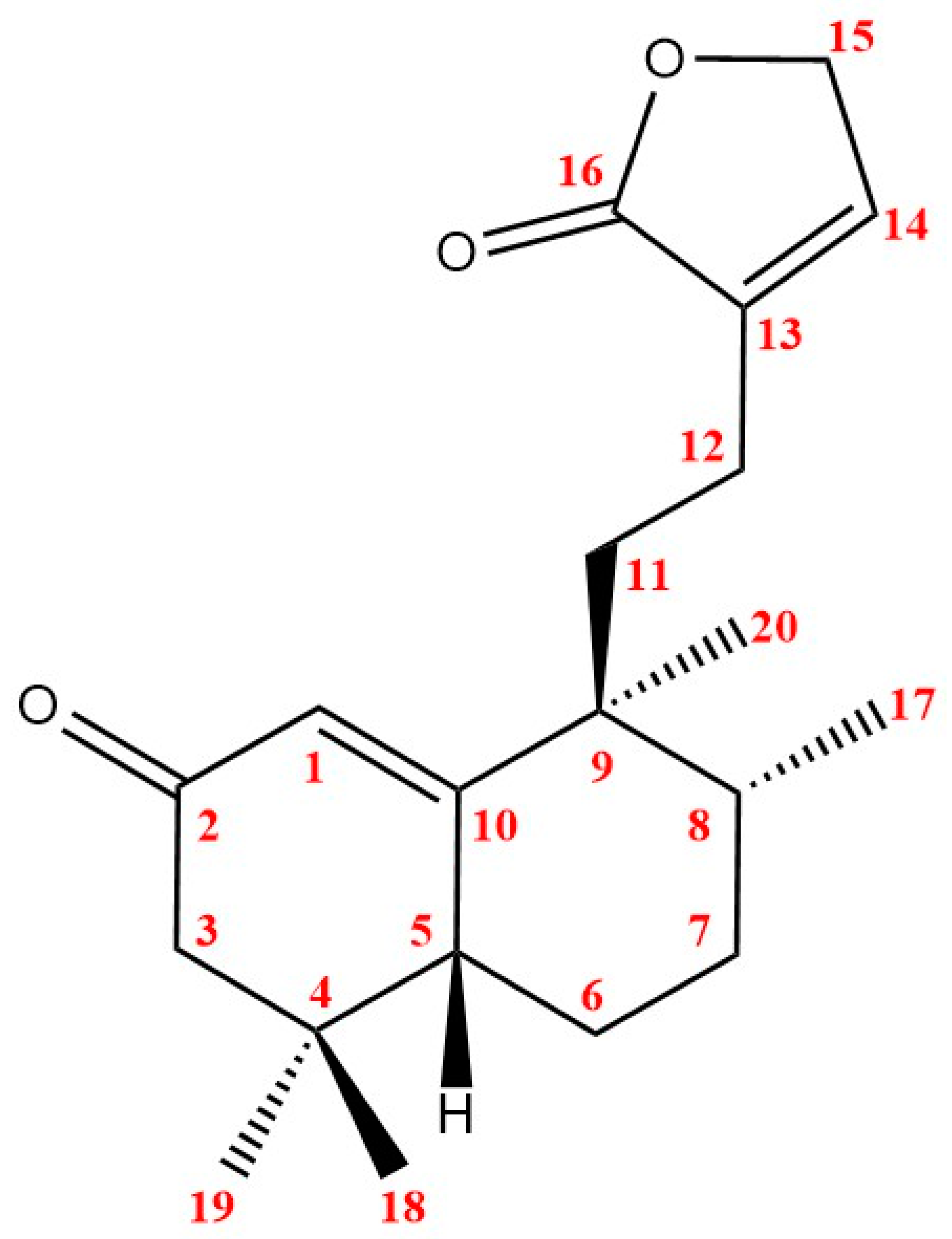
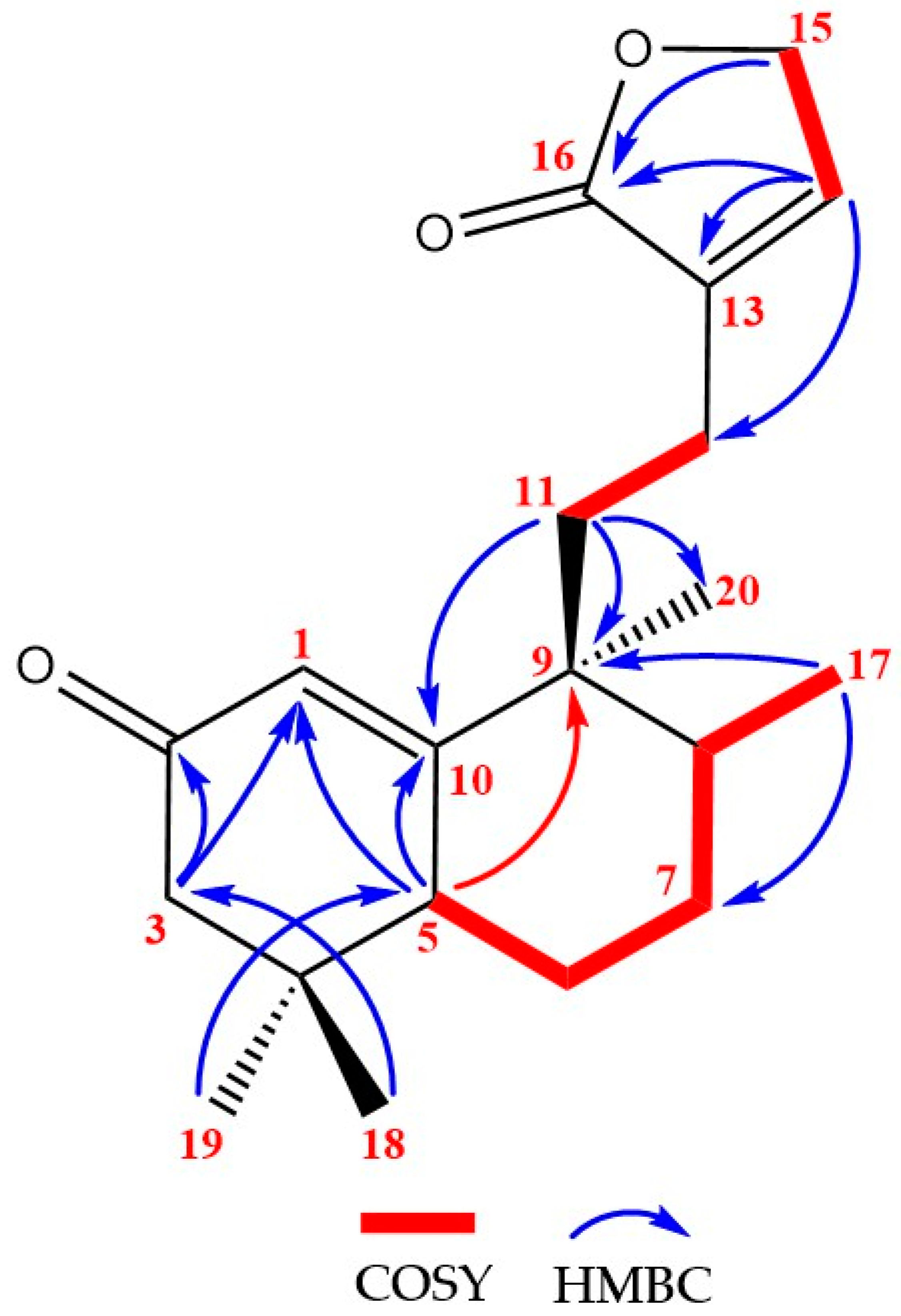
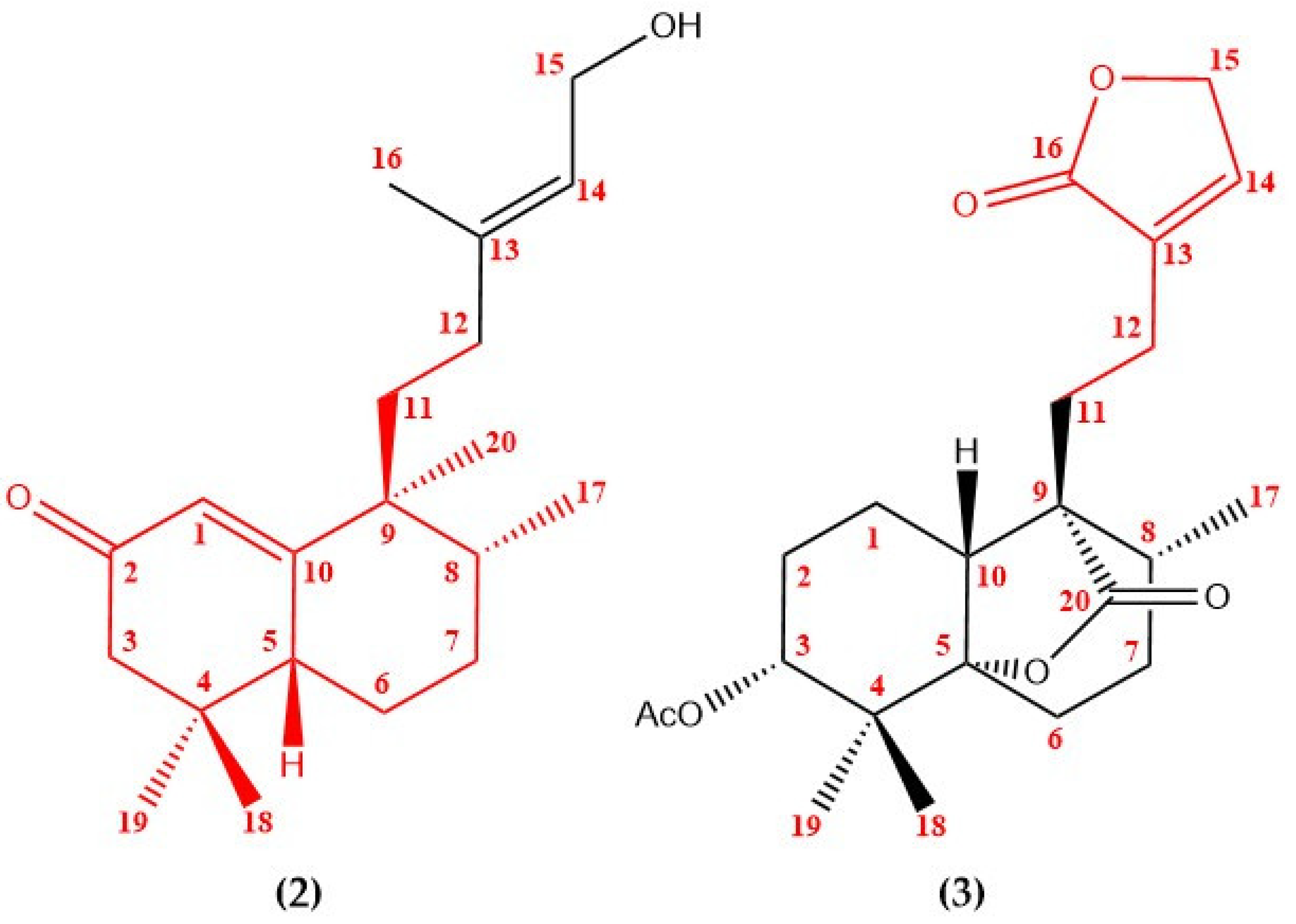
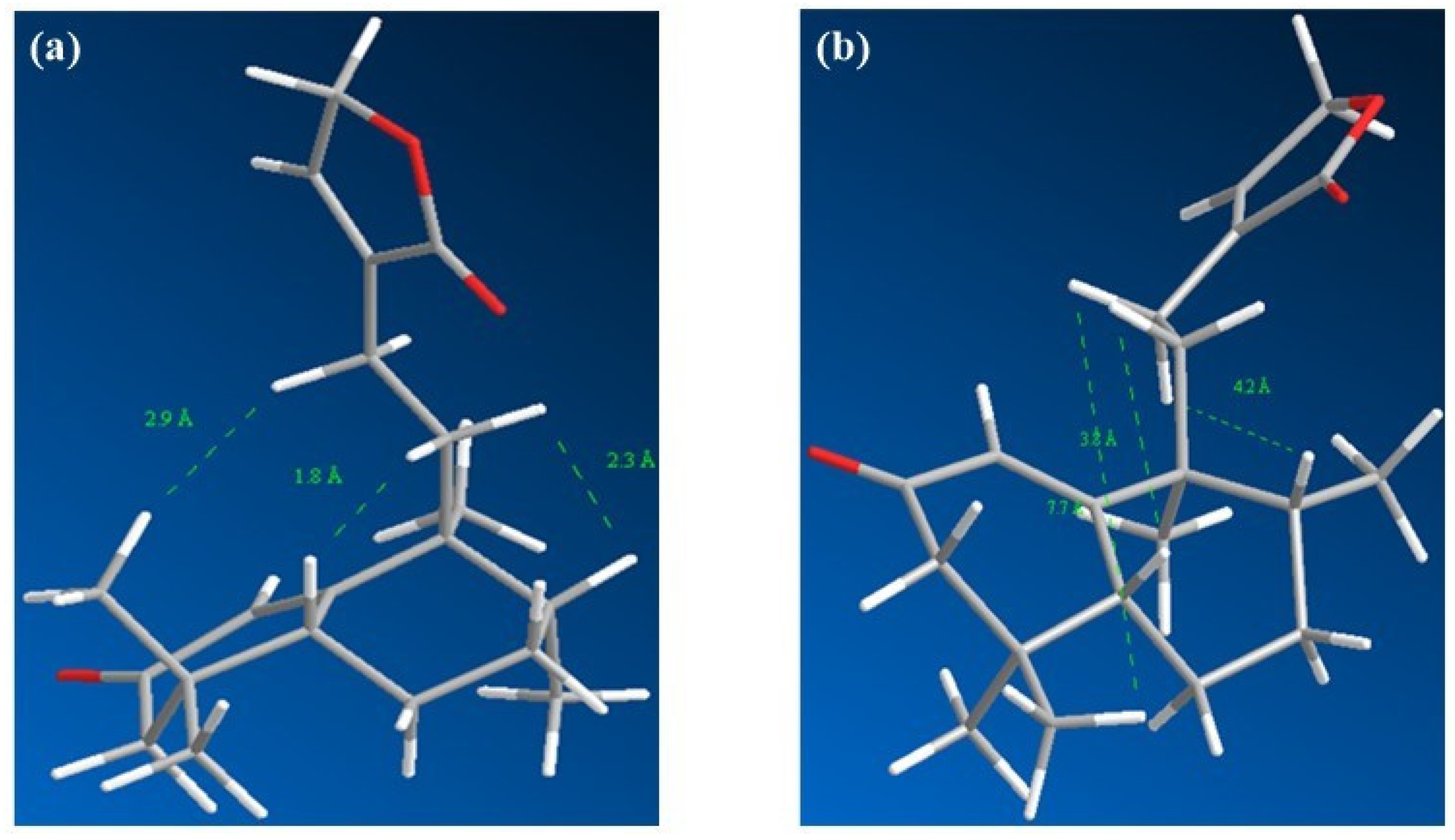
| Position | δC/ppm, Type | δH/ppm (Integral, Multiplicity, J/Hz) |
|---|---|---|
| 1 | 125.1, CH | 5.8 (1H, s) |
| 2 | 202.7, C | |
| 3 | 49.6, CH2 | 2.35 (1H, d, 15.5); 2.09 (1H, d, 15.5) |
| 4 | 35.5, C | |
| 5 | 46.4, CH | 2.45 (1H, dd, 12.8, 4.7) |
| 6 | 24.8, CH2 | 1.86 (1H, m); 1.59 (1H, m) |
| 7 | 29.2, CH2 | 2.16 (1H, m); 1.47 (1H, m) |
| 8 | 41.7, CH | 1.84 (1H, m) |
| 9 | 46.3, C | |
| 10 | 173.1, C | |
| 11 | 38.2, CH2 | 2.30 (1H, m); 1.50 (1H, m) |
| 12 | 21.4, CH2 | 2.27 (1H, m); 1.92 (1H, m) |
| 13 | 134.1, C | |
| 14 | 148.0, CH | 7.37 (1H, s) |
| 15 | 72.2, CH2 | 4.81 (2H, s) |
| 16 | 176.9, C | |
| 17 | 15.9, CH3 | 0.87 (3H, d, 7.0) |
| 18 | 28.6, CH3 | 1.03 (3H, s) |
| 19 | 25.9, CH3 | 0.99 (3H, s) |
| 20 | 21.8, CH3 | 1.11 (3H, s) |
| Position | 13C Shifts of 1 in CD3OD-d4, Type | Reference 13C Shifts of 2 in CDCl3-d1 [6], Type | Reference 13C Shifts of 3 in CDCl3-d1 [7], Type |
|---|---|---|---|
| 1 | 125.1, CH | 122.8, CH * | 18.3, CH2 |
| 2 | 202.7, C | 199.9, C * | 23.7, CH2 |
| 3 | 49.6, CH2 | 48.5, CH2 * | 76.0, CH |
| 4 | 35.5, C | 34.5, C * | 37.9, C |
| 5 | 46.4, CH | 45.3, CH * | 86.4, C |
| 6 | 24.8, CH2 | 23.8, CH2 * | 29.7, CH2 |
| 7 | 29.2, CH2 | 34.0, CH2 * | 28.9, CH2 |
| 8 | 41.7, CH | 40.3, CH * | 33.4, CH |
| 9 | 46.3, C | 44.9, C * | 55.3, C |
| 10 | 173.1, C | 169.9, C * | 46.3, CH |
| 11 | 38.2, CH2 | 37.8, CH2 * | 24.2, CH2 * |
| 12 | 21.4, CH2 | 28.3, CH2 * | 19.5, CH2 * |
| 13 | 134.1, C | 139.8, C | 133.5, C * |
| 14 | 148.0, CH | 124.4, CH | 144.1, CH * |
| 15 | 72.2, CH2 | 59.3, CH2 | 70.4, CH2 * |
| 16 | 176.9, C | 15.6, CH3 | 174.0, C * |
| 17 | 15.9, CH3 | 21.5, CH3 * | 16.0, CH3 |
| 18 | 28.6, CH3 | 25.9, CH3 * | 23.9, CH3 |
| 19 | 25.9, CH3 | 28.5, CH3 * | 20.1, CH3 |
| 20 | 21.8, CH3 | 17.8, CH3 * | 177.5, C |
| Ac | 170.6, C 21.1, CH3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajakulendran, J.E.; Oluwabusola, E.T.; Ebel, R.; Jaspars, M. Fosbergenone: 3-[2-(1,2,5,5-Tetramethyl-7-oxo-1,2,3,4,4a,5,6,7-octahydronaphthalen-1-yl)ethyl]-2,5-dihydrofuran-2-one. Molbank 2022, 2022, M1391. https://doi.org/10.3390/M1391
Rajakulendran JE, Oluwabusola ET, Ebel R, Jaspars M. Fosbergenone: 3-[2-(1,2,5,5-Tetramethyl-7-oxo-1,2,3,4,4a,5,6,7-octahydronaphthalen-1-yl)ethyl]-2,5-dihydrofuran-2-one. Molbank. 2022; 2022(2):M1391. https://doi.org/10.3390/M1391
Chicago/Turabian StyleRajakulendran, Joy Ebenezer, Emmanuel T. Oluwabusola, Rainer Ebel, and Marcel Jaspars. 2022. "Fosbergenone: 3-[2-(1,2,5,5-Tetramethyl-7-oxo-1,2,3,4,4a,5,6,7-octahydronaphthalen-1-yl)ethyl]-2,5-dihydrofuran-2-one" Molbank 2022, no. 2: M1391. https://doi.org/10.3390/M1391
APA StyleRajakulendran, J. E., Oluwabusola, E. T., Ebel, R., & Jaspars, M. (2022). Fosbergenone: 3-[2-(1,2,5,5-Tetramethyl-7-oxo-1,2,3,4,4a,5,6,7-octahydronaphthalen-1-yl)ethyl]-2,5-dihydrofuran-2-one. Molbank, 2022(2), M1391. https://doi.org/10.3390/M1391







