Synthesis and Structure Determination of 1-(4-Methoxyphenyl)-5-methyl-N’-(2-oxoindolin-3-ylidene)-1H-1,2,3-triazole-4-carbohydrazide
Abstract
:1. Introduction
2. Results and Discussion
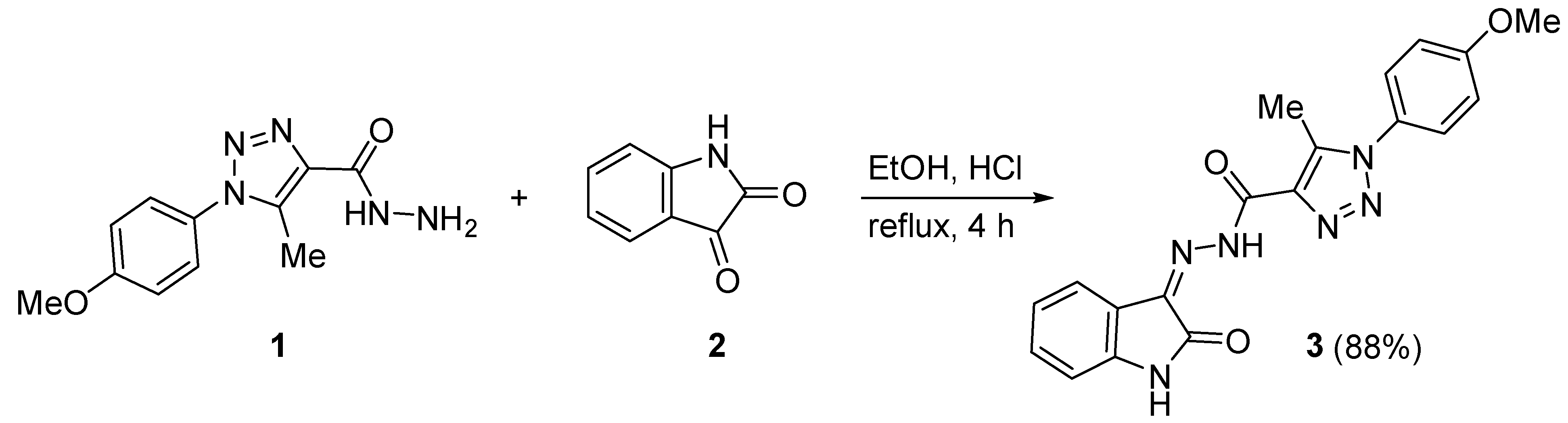
2.1. Synthesis of 3
2.2. NMR Spectroscopy
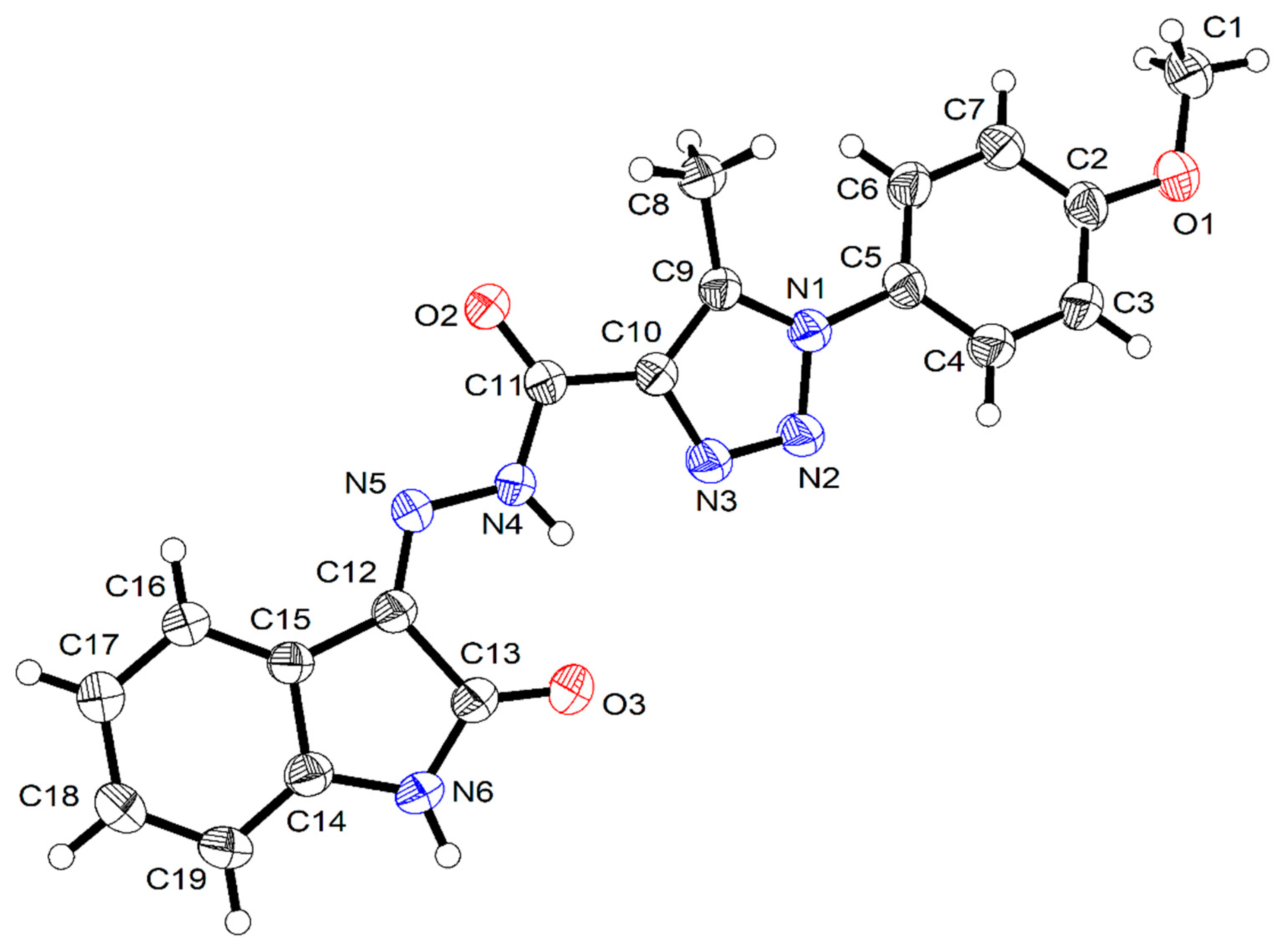
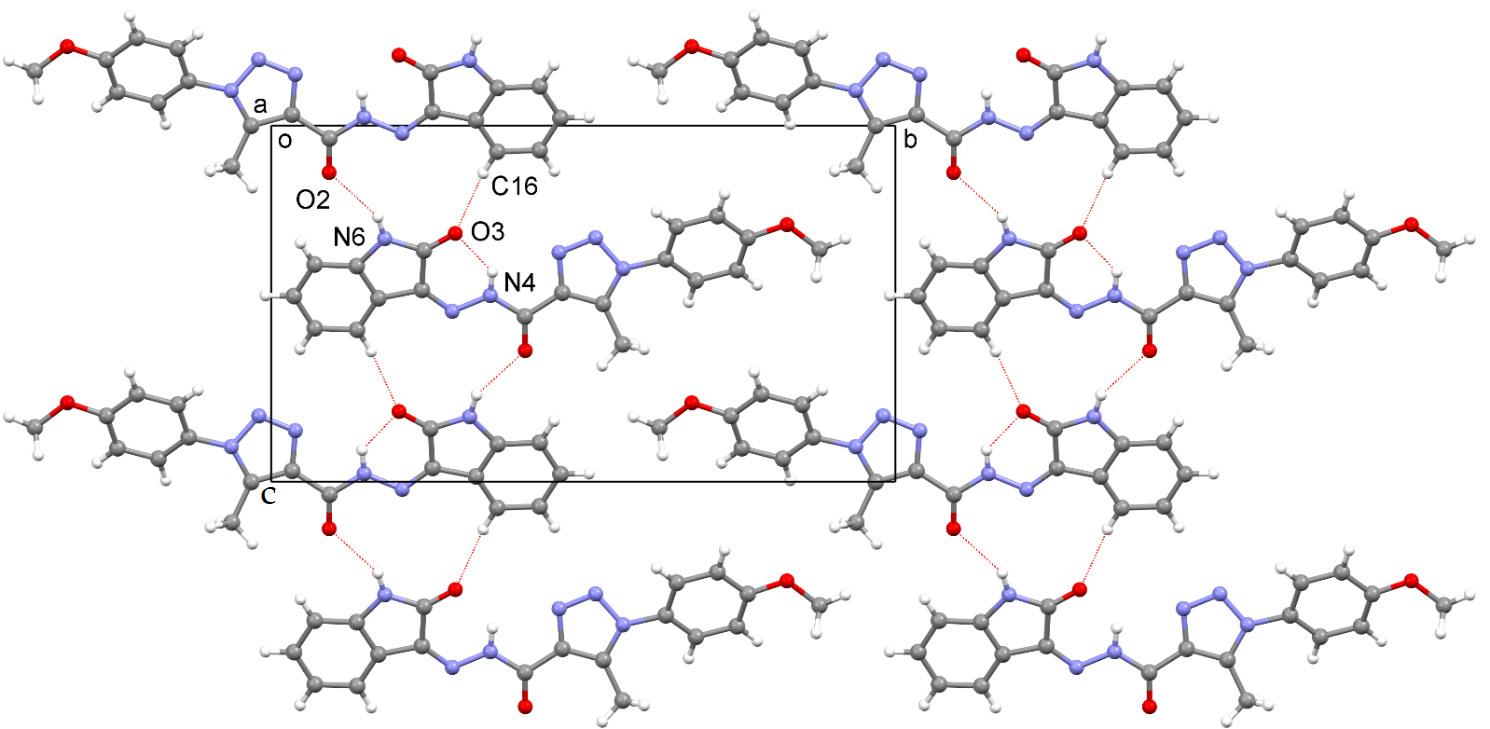
2.3. X-ray Structure
3. Materials and Methods
3.1. General
3.2. Synthesis of 3
3.3. Data Collection and Refinement Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gribanov, P.S.; Philippova, A.N.; Topchiy, M.A.; Minaeva, L.I.; Asachenko, A.F.; Osipov, S.N. General method of synthesis of 5-(het)arylamino-1,2,3-triazoles via Buchwald–Hartwig reaction of 5-amino-or 5-halo-1,2,3-triazoles. Molecules 2022, 27, 1999. [Google Scholar] [CrossRef] [PubMed]
- Topchiy, M.A.; Zharkova, D.A.; Asachenko, A.F.; Muzalevskiy, V.M.; Chertkov, V.A.; Nenajdenko, V.G.; Nechaev, M.S. Mild and Regioselective Synthesis of 3-CF3-Pyrazoles by the AgOTf-Catalysed Reaction of CF3-Ynones with Hydrazines. Eur. J. Org. Chem. 2018, 2018, 3750–3755. [Google Scholar] [CrossRef]
- Huang, W.; Buchwald, S.L. Palladium-Catalyzed N-Arylation of Iminodibenzyls and Iminostilbenes with Aryl- and Heteroaryl Halides. Chem. Eur. J. 2016, 22, 14186–14189. [Google Scholar] [CrossRef] [PubMed]
- Lannes, A.C.; Leal, B.; Novais, J.S.; Lione, V.; Monteiro, G.C.T.S.; Lourenço, A.L.; Sathler, P.C.; Jordão, A.K.; Rodrigues, C.R.; Cabral, L.M.; et al. Exploring N-acylhydrazone derivatives against clinical resistant bacterial strains. Curr. Microbiol. 2014, 69, 357–364. [Google Scholar] [CrossRef]
- He, J.-B.; Feng, L.-L.; Li, J.; Tao, R.-J.; Ren, Y.-L.; Wan, J.; He, H.-W. Design, synthesis and molecular modeling of novel N-acylhydrazone derivatives as pyruvate dehydrogenase complex E1 inhibitors. Bioorg. Med. Chem. 2014, 22, 89–94. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Alotaibi, M.H.; El-Hiti, G.A. Synthesis of new symmetrical N,N’-diacylhydrazines and 2-(1,2,3-triazol-4-yl)-1,3,4-oxadiazoles. Lett. Org. Chem. 2017, 14, 591–596. [Google Scholar] [CrossRef]
- Jadhav, R.P.; Raundal, U.N.; Patil, A.A.; Bobade, V.D. Synthesis and biological evaluation of a series of 1,4-disubstituted 1,2,3-triazole derivatives as possible antimicrobial agents. J. Saudi Chem. Soc. 2017, 21, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Johansson, J.R.; Beke-Somfai, T.; Said Stalsmeden, A.; Kann, N. Ruthenium-catalyzed azide alkyne cycloaddition reaction: Scope, mechanism, and applications. Chem. Rev. 2016, 116, 14726–14768. [Google Scholar] [CrossRef] [Green Version]
- Ashok, D.; Chiranjeevi, P.; Kumar, A.V.; Sarasija, M.; Krishna, V.S.; Sriram, D.; Balasubramanian, S. 1,2,3-Triazole-fused spirochromenes as potential anti-tubercular agents: Synthesis and biological evaluation. RSC Adv. 2018, 8, 16997–17007. [Google Scholar] [CrossRef] [Green Version]
- Emileh, A.; Duffy, C.; Holmes, A.P.; Rosemary Bastian, A.; Aneja, R.; Tuzer, F.; Rajagopal, S.; Li, H.; Abrams, C.F.; Chaiken, I.M. Covalent conjugation of a peptide triazole to HIV-1 gp120 enables intramolecular binding site occupancy. Biochemistry 2014, 53, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Krishna, P.M.; Ramachary, D.B.; Peesapati, S. Azide–acetonitrile “click” reaction triggered by Cs2CO3: The atom-economic, high-yielding synthesis of 5-amino-1,2,3-triazoles. RSC Adv. 2015, 5, 62062–62066. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Matiychuk, V.S.; Obushak, N.B. Synthesis of 1H-1,2,3-triazole derivatives by the cyclization of aryl azides with 2-benzothiazolylacetonone, 1,3-benzo-thiazol-2-ylacetonitrile, and (4-aryl-1,3-thiazol-2-yl)acetonitriles. Chem. Heterocycl. Compd. 2009, 45, 483–488. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Liu, G.; Xu, H.; Song, L.; Chen, J.; Li, J.; Zhang, Z. Transition-metal-free synthesis of 5-amino-1,2,3-triazoles via nucleophilic addition/cyclization of carbodiimides with diazo compounds. Org. Chem. Front. 2021, 8, 599–604. [Google Scholar] [CrossRef]
- Opsomer, T.; Thomas, J.; Dehaen, W. Chemoselectivity in the synthesis of 1,2,3-triazoles from enolizable ketones, primary alkylamines, and 4-nitrophenyl azide. Synthesis 2017, 49, 4191–4198. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Sengupta, S.; Petersen, J.L.; Akhmedov, N.G.; Shi, X. Triazole-Au(I) complexes: A new class of catalysts with improved thermal stability and reactivity for intermolecular alkyne hydroamination. J. Am. Chem. Soc. 2009, 131, 12100–12102. [Google Scholar] [CrossRef]
- Gribanov, P.S.; Atoian, E.M.; Philippova, A.N.; Topchiy, M.A.; Asachenko, A.F.; Osipov, S.N. One-pot synthesis of 5-amino-1,2,3-triazole derivatives via dipolar azide–nitrile cycloaddition and Dimroth rearrangement under solvent-free conditions. Eur. J. Org. Chem. 2021, 2021, 1378–1384. [Google Scholar] [CrossRef]
- Gribanov, P.S.; Topchiy, M.A.; Karsakova, I.V.; Chesnokov, G.A.; Smirnov, A.Y.; Minaeva, L.I.; Asachenko, A.F.; Nechaev, M.S. General method for the synthesis of 1,4-disubstituted 5-halo-1,2,3-triazoles. Eur. J. Org. Chem. 2017, 2017, 5225–5230. [Google Scholar] [CrossRef]
- Nath, R.; Pathania, S.; Grover, G.; Akhtar, M.J. Isatin containing heterocycles for different biological activities: Analysis of structure activity relationship. J. Mol. Struct. 2020, 1222, 128900. [Google Scholar] [CrossRef]
- de Paiva, R.E.F.; Vieira, E.G.; da Silva, D.R.; Wegermann, C.A.; Ferreira, A.M.C. Anticancer compounds based on isatin-derivatives: Strategies to ameliorate selectivity and efficiency. Front. Mol. Biosci. 2020, 7, 627272. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Du, H.-Z.; Liu, H.-L.; He, Q.-S.; Xu, Z. Isatin dimers and their biological activities. Arch. Pharm. 2020, 353, e1900299. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.H.; Abdel-Wahab, B.F.; Yousif, E.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O. Z. Kristallogr. NCS 2020, 235, 479–481. [Google Scholar] [CrossRef] [Green Version]
- Alotaibi, M.H.; Mohamed, H.A.; Abdel-Wahab, B.F.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. Crystal structure of N’-(1-(2-hydroxyphenyl)ethylidene)-5-methyl-1-phenyl-1H-1,2,3-triazole-4-carbohydrazide, C18H17N5O2. Z. Kristallogr. NCS 2019, 234, 355–357. [Google Scholar] [CrossRef]
- Geesi, M.H.; Mohamed, H.A.; Abdel-Wahab, B.F.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. Crystal structure of ethyl 4-amino-5-(5-methyl-1-(4-tolyl)-1H-1,2,3-triazole-4-carbonyl)-2-(phenylamino)thiophene-3-carboxylate, C24H23N5O3S. Z. Kristallogr. NCS 2018, 233, 673–674. [Google Scholar] [CrossRef]
- Alotaibi, M.H.; Mohamed, H.A.; Abdel-Wahab, B.F.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. Synthesis and structure elucidation of N′-(4-methoxybenzylidene)-5-methyl-1-phenyl-1H-1,2,3-triazole-4-carbohydrazide. Molbank 2018, 2018, M1034. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.-H.; Hui, X.-P.; Zhang, Y.; Zhang, Z.-Y.; Li, Z.-C.; Liao, R.-A. Synthesis and antifungal activities of ω-[4-aryl-5-(1-phenyl-5-methyl-1,2,3-triazol-4-yl)-1,2,4-triazol-3-thio]-ω-(1H-1,2,4-triazol-1-yl)acetophenones. J. Chin. Chem. Soc. 2001, 48, 121–125. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariuki, B.M.; Abdel-Wahab, B.F.; Farahat, A.A.; El-Hiti, G.A. Synthesis and Structure Determination of 1-(4-Methoxyphenyl)-5-methyl-N’-(2-oxoindolin-3-ylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molbank 2022, 2022, M1374. https://doi.org/10.3390/M1374
Kariuki BM, Abdel-Wahab BF, Farahat AA, El-Hiti GA. Synthesis and Structure Determination of 1-(4-Methoxyphenyl)-5-methyl-N’-(2-oxoindolin-3-ylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molbank. 2022; 2022(2):M1374. https://doi.org/10.3390/M1374
Chicago/Turabian StyleKariuki, Benson M., Bakr F. Abdel-Wahab, Abdelbasset A. Farahat, and Gamal A. El-Hiti. 2022. "Synthesis and Structure Determination of 1-(4-Methoxyphenyl)-5-methyl-N’-(2-oxoindolin-3-ylidene)-1H-1,2,3-triazole-4-carbohydrazide" Molbank 2022, no. 2: M1374. https://doi.org/10.3390/M1374
APA StyleKariuki, B. M., Abdel-Wahab, B. F., Farahat, A. A., & El-Hiti, G. A. (2022). Synthesis and Structure Determination of 1-(4-Methoxyphenyl)-5-methyl-N’-(2-oxoindolin-3-ylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molbank, 2022(2), M1374. https://doi.org/10.3390/M1374






