Synthesis of 8-Aminoquinoline Amides of Ursonic and Oleanonic Acid
Abstract
:1. Introduction
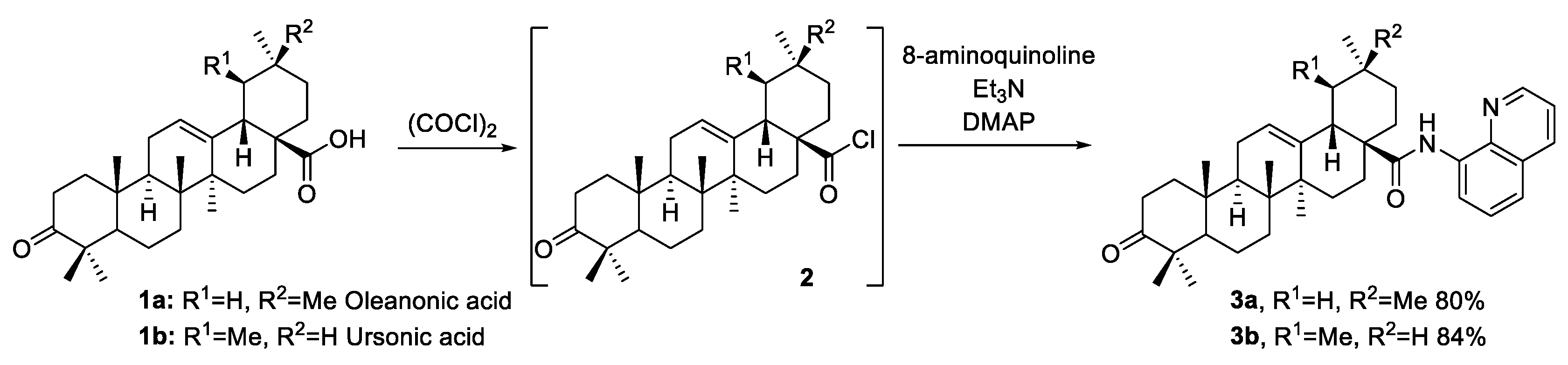
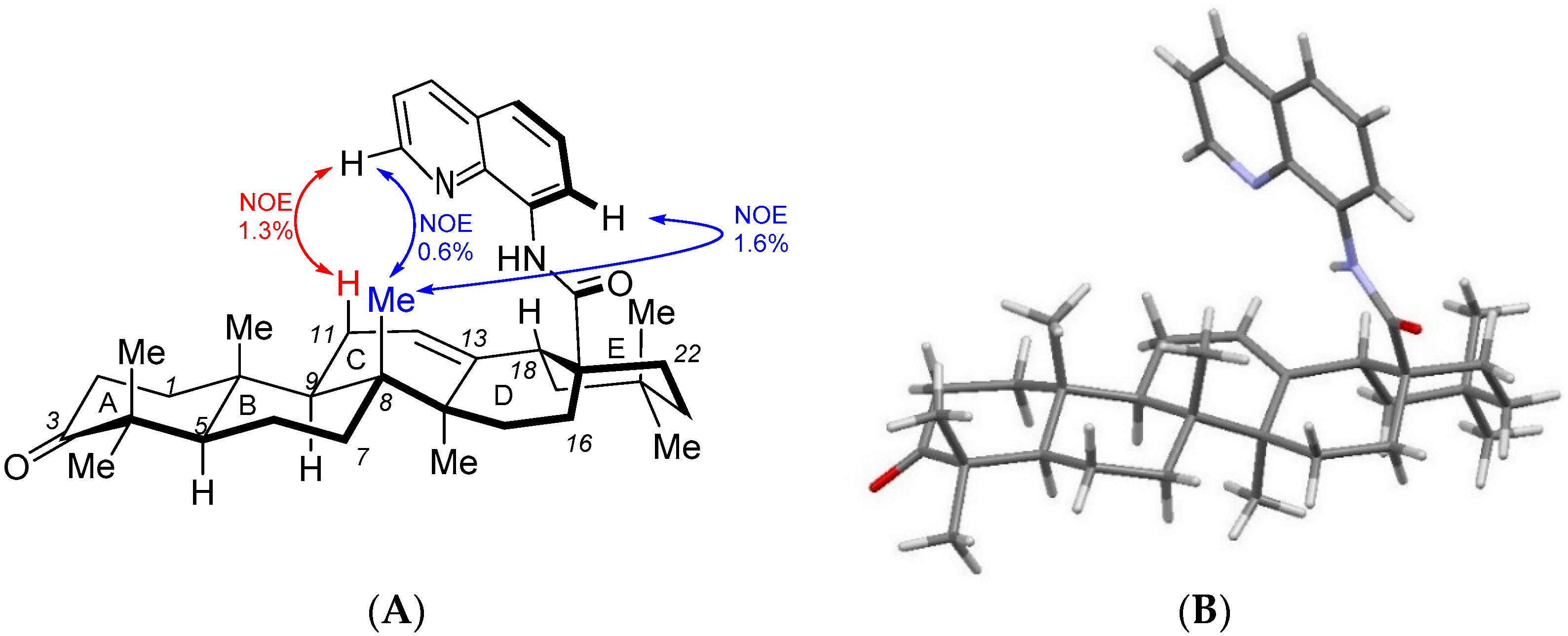
2. Results and Discussion
3. Materials and Methods
- 3-Oxo-olean-12-en-28-oic acid 8-aminoquinoline amide3a. Yield of 80% (1.321 g) as a white amorphous solid. Single crystals of amide 3a, which are suitable for X-ray analysis, were obtained by slow evaporation from DCM/hexane’s mixture with m.p. 238–239 °C. Rf = 0.42 (Hex/EtOAc 4:1). 1H-NMR (500 MHz, CDCl3) δ 10.37 (s, 1H, H-N), 8.85 (dd, 3J = 7.7 Hz, 4J = 1.7 Hz, 1H, H-C(7′)), 8.81 (dd, 3J = 4.2 Hz, 4J = 1.7 Hz, 1H,H-C(2′)), 8.15 (dd, 3J = 8.2 Hz, 4J = 1.7 Hz, 1H, H-C(4′)), 7.52 (dd, 3J = 8.3, 7.7 Hz, 1H, H-C(6′)), 7.47 (dd, 3J = 8.3 Hz, 4J = 1.7 Hz, 1H, H-C(5′)), 7.45 (dd, 3J = 8.2 Hz, 3J = 4.2 Hz, 1H, H-C(3′)), 5.73 (t, 3J = 3.7 Hz, 1H, H-C(12)), 3.01 (dd, 3J = 12.9 Hz, 4J = 3.7 Hz, 1H, H-C(18)), 2.50 (ddd, 2J = 15.9 Hz, 3J = 11.1, 7.3 Hz, 1H, Ha-C(2)), 2.34 (ddd, 2J = 15.9 Hz, 3J = 6.8, 3.7 Hz, 1H, Hb-C(2)), 2.16 (td, 2J = 13.5 Hz,3J = 3.7 Hz, 1H, Ha-C(16)), 1.97 (m, 2H, H2-C(11)), 1.92–1.79 (m, 5H, Hb-C(16), Ha-C(1), Ha-C(19), H2C(22)), 1.74 (ddd, 2J = 14.1 Hz, 3J = 13.3, 4.2 Hz, 1H, Ha-C(15)), 1.66 (dd, 3J = 9.0, 8.6 Hz, 1H, H-C(9)), 1.52–1.23 (m, 9H, H-C(5),H2C(7), Hb-C(1), H2-C(6), Hb-C(19), H2C(21)), 1.22 (s, 3H, H3-C(27)), 1.13 (ddd, 2J = 14.1 Hz, 3J = 6.7, 3.7 Hz, 1H, Hb-C(15)), 1.04 (s, 3H, H3-C(23)), 0.99 (s, 3H, H3-C(29)), 0.96 (s, 3H, H3-C(30)), 0.95 (s, 3H, H3-C(24)), 0.85 (s, 3H, H3-C(25), 0.54 (s, 3H, H3-C(26)). 13C-NMR (125.6 MHz, CDCl3) δ 217.71 (C3), 176.95(O=C-NH), 147.84(C2′) 143.25 (C13), 139.02(C8a′), 136.23 (C4′), 134.94 C(8′), 127.98 (C4a′), 127.56(C6′), 123.87(C12), 121.44 (C3′), 121.13 (C5′), 116.38(C7′), 55.25 (C5), 48.13 (C17), 47.42 (C4), 46.89 (C9), 46.77 (C19), 42.27 (C18), 41.98 (C14), 39.43 (C8), 39.19 (C1), 36.63 (C10), 34.32 (C21), 34.15 (C2), 33.11 (C30), 32.97 (C22), 31.96 (C7), 30.81 (C20), 27.58 (C15), 26.40 (C23), 25.88 (C27), 24.15 (C16), 23.70 (C29), 23.61 C(11), 21.40 (C24), 19.47 (C6), 16.23 (C26), 15.00 (C25). IR (FTIR): 3436 (s), 3333 (s), 2942 (s), 2863 (m), 1702 (s), 1673 (s), 1532 (s), 1487 (m), 1462 (m), 1424 (m), 1384 (m), 1326 (m), 1261 (w), 1164 (m), 1074 (w), 999 (w), 826 (m), 792 (m), 771 (w), 678 (w) cm−1. HRMS (ESI): m/z calcd. for [C39H52N2O2+H]+ 581.4107; found 581.4116.
- 3-Oxo-urs-12-en-28-oic acid 8-aminoquinoline amide3b. Yield of 84% (1.385 g) as a white amorphous solid. Rf = 0.40 (Hex/EtOAc 4:1). 1H-NMR (500 MHz, CDCl3) δ 10.30 (s, 1H,N-H), 8.85 (dd, 3J = 7.7 Hz, 4J = 1.5 Hz, 1H,H-C(7′)), 8.82 (dd, 3J = 4.0 Hz, 4J = 1.5 Hz, 1H, H-C(2′)), 8.15 (dd, 3J = 8.2 Hz, 4J = 1.5 Hz, 1H, H-C(4′)), 7.53 (dd,3J = 8.1, 7.7 Hz, 1H, H-C(6′)), 7.49 (dd 3J = 8.1 Hz, 4J = 1.5 Hz, 1H, H-C(5′), 7.47 (dd, 3J = 8.2, 4.0 Hz, 1H, H-C(3′)), 5.70 (t, 3J = 3.8 Hz, 1H, H-C(12)), 2.51 (ddd, 2J = 15.9 Hz 3J = 10.9, 7.3 Hz, 1H, Ha-C(2)), 2.41–2.34 (m, 2H, Hb-C(2), H-C(18)), 2.17 (td, 2J = 13.7 Hz, 3J = 4.3 Hz, 1H, Ha-C(16)), 2.09–1.81 (m, 6H, Ha-C(1), Ha-C(15), Hb-C(16), H2C-(11), Ha-C(22)), 1.70 (ddd, 2J = 13.8 Hz, 3J = 13.5, 4.1 Hz, 1H, Hb-C(22)), 1.67–1.26 (m, 10H, Hb-C(1), H-C(5), H2-C(6), H2-C(7), H2-C(21), H-C(19), H-C(9)), 1.18 (s, 3H, H3-C(27)), 1.17–1.08 (m, 2H, Hb-C(15), H-C(20)), 1.07 (s, 3H, H3-C(23)), 1.03 (s, 3H, H3-C(30)), 1.01 (s, 3H, H3-C(29)), 0.97 (s, 3H, H3-C(24)), 0.83 (s, 3H, H3-C(25)), 0.54 (s, 3H, H3-C(26)). 13C-NMR (125.6 MHz, CDCl3) δ 217.65(C3), 176.74 (O=C-NH), 147.70(C1′), 138.94 (C8a′), 137.75(C13), 136.14(C4′), 134.89 (C8′), 127.87(C4a′), 127.46 (C6′), 126.89 (C3′), 121.31 (C12), 120.96 (C5′), 116.31 (C7′), 55.09 (C5), 53.70 (C18), 49.43 (C17), 47.27 (C4), 46.68 (C9), 42.21 (C14), 39.81 (C19), 39.44 (C8), 39.19 (C1), 38.94 (C20), 37.38 (C22), 36.45 (C10), 34.04 (C2), 32.10 (C7), 30.95 (C21), 27.89 (C15), 26.39 (C23), 25.08 (C16), 23.45 (C27), 23.29 (C11), 21.29 (C24), 21.15 (C23), 19.36 (C6), 17.17 (C29), 16.19 (C26), 15.03 (C25). IR (FTIR): 3367 (s), 2927 (s), 2868 (s), 1705 (s), 1668 (s), 1526 (s), 1486 (s), 1458 (m), 1424 (m), 1383 (s), 1324 (m), 826 (m), 792 (m), 671(w), 663 (w) cm−1. HRMS (ESI): m/z calcd. for [C39H52N2O2+H]+ 581.4107; found 581.4124.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeung, M.F. A review on presence of oleanolic acid in natural products. Nat. Prod. Med. 2009, 2, 77–290. [Google Scholar]
- Ramsay, K.S.; Wafo, P.; Ali, Z.; Khan, A.; Oluyemisi, O.O.; Marasini, B.P.; Khan, I.A.; Bonaventure, N.T.; Choudhary, M.I.; Atta-ur-Rahman. Chemical con-stituents of Stereospermum acuminatissimum and their urease and alpha-chymotrypsin inhibitions. Fitoterapia 2012, 83, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; You, H.J.; Jeong, H.G. Nitric oxide and tumor necrosis factor-a production by oleanolic acid via nuclear factor-kB activation in macrophages. Biochem. Biophys. Res. Commun. 2001, 288, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.L.; Huang, C.Y.; Wu, S.T.; Yin, M.C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Mieriņa, I.; Vilskersts, R.; Turks, M. Delivery Systems for Birch-bark Triterpenoids and their derivatives in anticancer research. Curr. Med. Chem. 2020, 27, 1308–1336. [Google Scholar] [CrossRef]
- Lombrea, A.; Scurtu, A.D.; Avram, Z.; Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Dehelean, C.A.; Soica, C.; Danciu, C. Anticancer potential of betulonic acid derivatives. Int. J. Mol. Sci. 2021, 22, 3676. [Google Scholar] [CrossRef]
- Teodoro, T.; Zhang, L.; Alexander, T.; Yue, J.; Vranic, M.; Volchuk, A. Oleanolic acid enhances insulin secretion in pancreatic b-cells. FEBS Lett. 2008, 582, 1375–1380. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.M.; Yee, S.T.; Choi, J.; Choi, M.S.; Do, G.M.; Jeon, S.M.; Yeo, J.; Kim, M.J.; Seo, K.I.; Lee, M.K. Ursolic acid enhances the cellular immune system and pancreatic beta-cell function in streptozotocin-induced diabetic mice fed a high-fat diet. Int. Immunopharmacol. 2009, 9, 113–119. [Google Scholar] [CrossRef]
- Huguet, A.I.; del Carmen Recio, M.; Máñez, S.; Giner, R.M.; Ríos, J.L. Effect of triterpenoids on the inflammation induced by protein kinase C activators, neuronally acting irritants and other agents. Eur. J. Pharmacol. 2000, 410, 69–81. [Google Scholar] [CrossRef]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Kong, L.; Li, S.; Liao, Q.; Zhang, Y.; Sun, R.; Zhu, X.; Zhang, Q.; Wang, J.; Wu, X.; Fang, X.; et al. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013, 98, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cwynar, B.B. Anilides and Toluidides of 3β-Acetyloleanolic Acid. Nat. Prod. Commun. 2012, 7, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.W.; Dai, Y.C.; Xue, J.P.; Wang, J.C.; Lin, F.P.; Guo, Y.H. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Honda, Y.; Favaloro, F.G., Jr.; Gribble, G.W.; Suh, N.; Place, A.E.; Rendi, M.H.; Sporn, M.B. A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg. Med. Chem. Lett. 2002, 12, 1027–1030. [Google Scholar] [CrossRef]
- Li, J.F.; Zhao, Y.; Cai, M.M.; Li, X.F.; Li, J.X. Synthesis and evaluation of a novel series of heterocyclic oleanolic acid derivatives with anti-osteoclast formation activity. Eur. J. Med. Chem. 2009, 44, 2796–2806. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Shen, J.K.; Wang, H.K.; Cosentino, L.M.; Lee, K.H. Synthesis and anti-HIV activity of oleanolic acid derivatives. Bioorg. Med. Chem. Lett. 2011, 11, 3115–3118. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.X.; Zhao, J.; Wang, S.Z.; Pan, Y.; Tanaka, K.; Kadota, S. Synthesis and activity of oleanolic acid derivatives, a novel class of inhibitors of osteoclast formation. Bioorg. Med. Chem. Lett. 2005, 15, 1629–1632. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhang, W.; Hong, D.; Shi, L.; Shen, Q.; Li, J.Y.; Li, J.; Hu, L.H. Oleanolic acid and its derivatives: New inhibitor of protein tyrosine phosphatase 1B with cellular activities. Bioorg. Med. Chem. Lett. 2008, 16, 8697–8705. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Kuhfs, J.; Csuk, R. Selective killing of cancer cells with triterpenoic acid amides-The substantial role of an aromatic moiety alignment. Eur. J. Med. Chem. 2016, 122, 452–464. [Google Scholar] [CrossRef]
- Khan, A.Y.; Gopinatha, S.K. Natural isoquinoline alkaloids: Binding aspects to functional proteins, serum albumins, hemoglobin, and lysozyme. Biophys. Rev. 2015, 7, 407–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorka, A.P.; de Dios, A.; Roepe, P.D. Quinoline Drug–Heme Interactions and Implications for Antimalarial Cytostatic versus Cytocidal Activities. J. Med. Chem. 2013, 56, 5231–5246. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Sang, Y.L. Therapeutic Potential of Ursonic Acid: Comparison with Ursolic Acid. Biomolecules 2020, 10, 1505. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Z.; Hua, S.X.; Liao, Z.X.; Huang, X.C.; Wang, H.S. Side chain-functionalized aniline-derived ursolic acid derivatives as multidrug resistance reversers that block the nuclear factor-kappa B (NF-κB) pathway and cell proliferation. Med. Chem. Comm. 2017, 8, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.G.; Shabashov, D.; Daugulis, O. Highly regioselective arylation of sp3 C-H bonds catalyzed by palladium acetate. J. Am. Chem. Soc. 2005, 127, 13154–13155. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Sun, H.; Liu, J.; Cheng, K.; Zhang, P.; Zhang, L.; Hao, J.; Zhang, L.; Ni, P.; Zographos, S.E.; et al. Naturally Occurring Pentacyclic Triterpenes as Inhibitors of Glycogen Phosphorylase: Synthesis, Structure−Activity Relationships, and X-ray Crystallographic Studies. J. Med. Chem. 2008, 51, 3540–3554. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kroškins, V.; Lugiņina, J.; Mishnev, A.; Turks, M. Synthesis of 8-Aminoquinoline Amides of Ursonic and Oleanonic Acid. Molbank 2022, 2022, M1361. https://doi.org/10.3390/M1361
Kroškins V, Lugiņina J, Mishnev A, Turks M. Synthesis of 8-Aminoquinoline Amides of Ursonic and Oleanonic Acid. Molbank. 2022; 2022(2):M1361. https://doi.org/10.3390/M1361
Chicago/Turabian StyleKroškins, Vladislavs, Jevgeņija Lugiņina, Anatoly Mishnev, and Māris Turks. 2022. "Synthesis of 8-Aminoquinoline Amides of Ursonic and Oleanonic Acid" Molbank 2022, no. 2: M1361. https://doi.org/10.3390/M1361
APA StyleKroškins, V., Lugiņina, J., Mishnev, A., & Turks, M. (2022). Synthesis of 8-Aminoquinoline Amides of Ursonic and Oleanonic Acid. Molbank, 2022(2), M1361. https://doi.org/10.3390/M1361




