Ethyl 5-Formyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and DFT Calculation
Abstract
:1. Introduction
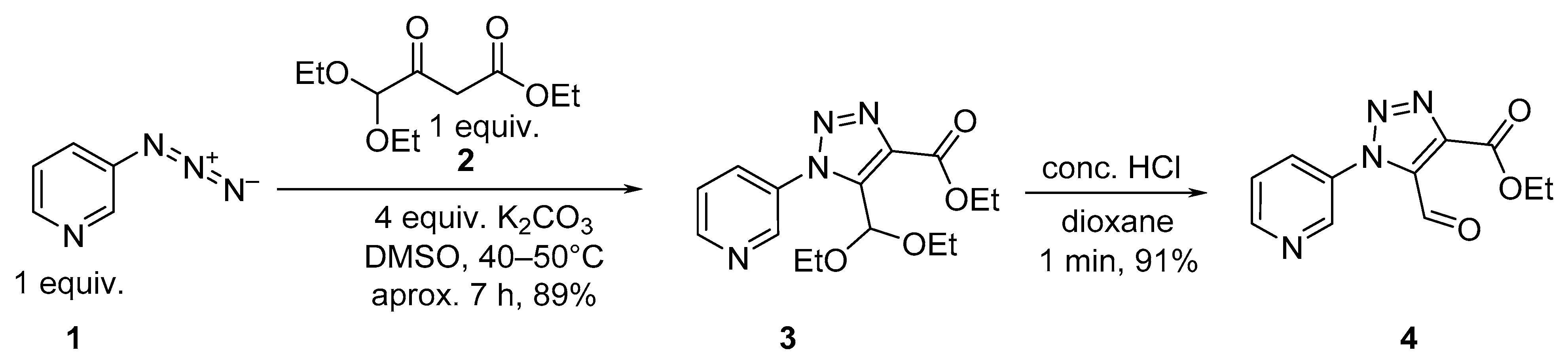
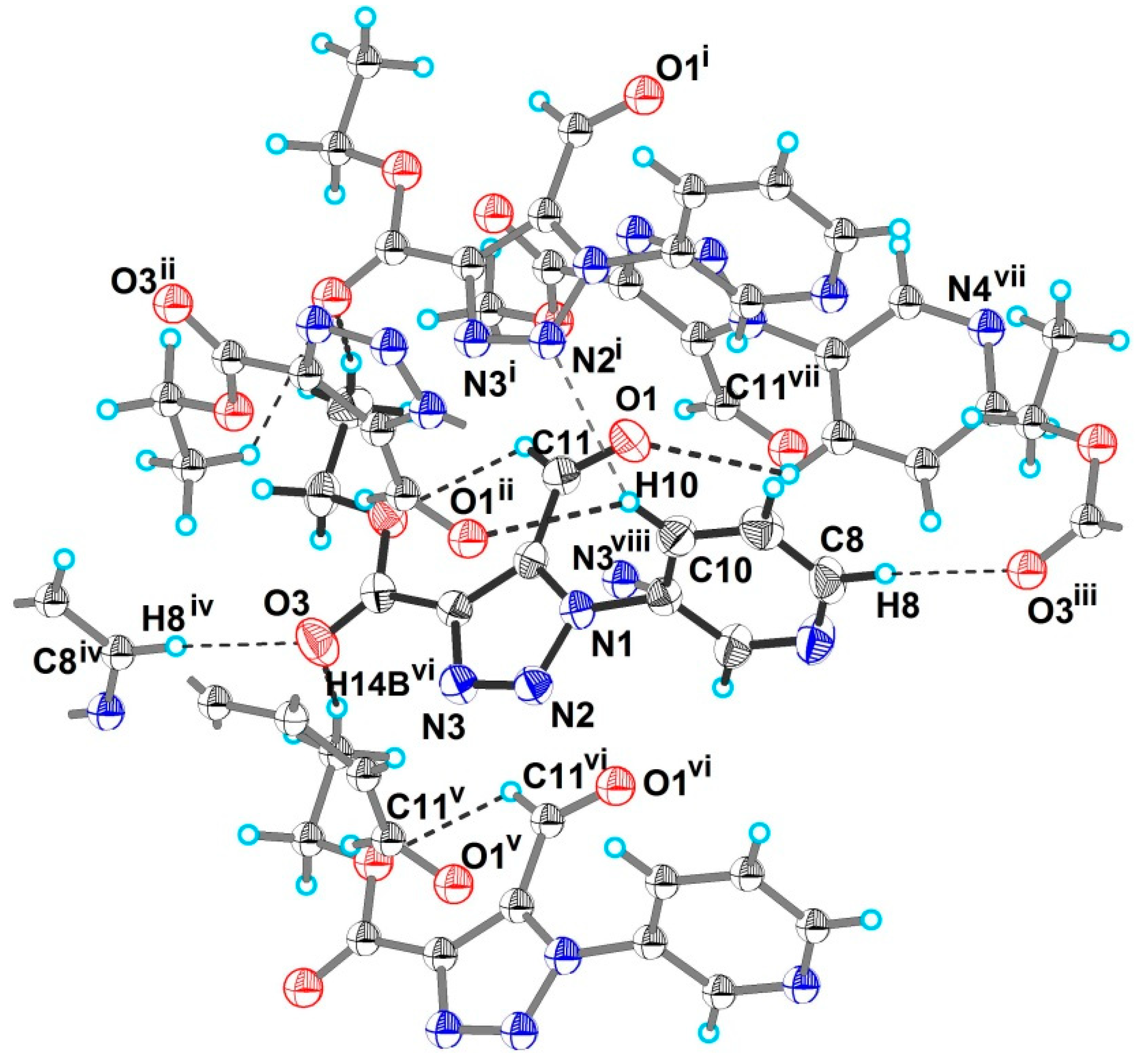
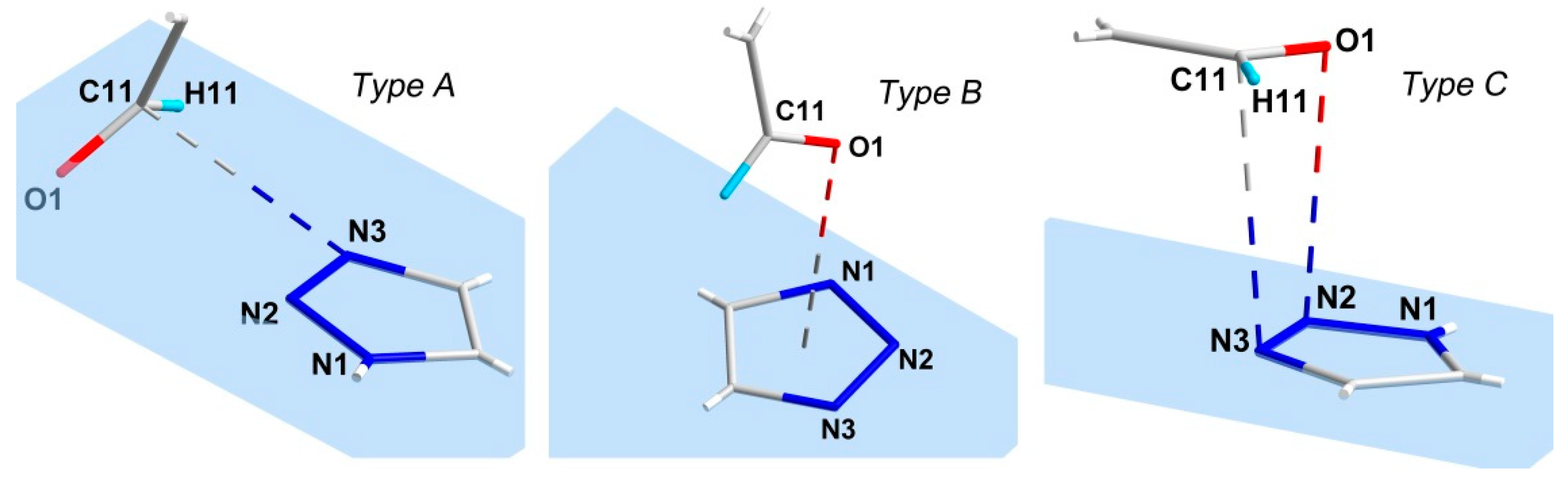
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of ethyl 5-(diethoxymethyl)-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate 3
3.2. Synthesis of ethyl 5-formyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate 4
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Li, Y.; Wang, F.; Ma, C.; Yang, G.; Yang, J.; Ren, J. p-Toluenesulfonic acid-catalyzed reaction of phthalaldehydic acids with difluoroenoxysilanes: Access to 3-difluoroalkyl phthalides. Synthesis 2021, 54, 161–170. [Google Scholar] [CrossRef]
- Ibrahimi, M.; Khoumeri, O.; Abderrahim, R.; Terme, T.; Vanelle, P. Synthesis of 3-benzylphthalide derivatives by using a TDAE strategy. Synlett 2021, 32, 283–286. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, D.-Q.; Zhang, J.-Y.; Yu, B.; Liu, H.-M. palladium-catalyzed ligand-free double cyclization reactions for the synthesis of 3-(1′-indolyl)-phthalides. Org. Lett. 2020, 22, 814–817. [Google Scholar] [CrossRef]
- Zou, Z.; Cai, G.; Chen, W.; Zou, C.; Li, Y.; Wu, H.; Chen, L.; Hu, J.; Li, Y.; Huang, Y. Metal-free cascade formation of intermolecular c-n bonds accessing substituted isoindolinones under cathodic reduction. J. Org. Chem. 2021, 86, 15777–15784. [Google Scholar] [CrossRef] [PubMed]
- Gartman, J.A.; Tambar, U.K. Total synthesis of (+)-Rubellin C. Org. Lett. 2020, 22, 9145–9150. [Google Scholar] [CrossRef]
- Gao, Z.; Qian, J.; Yang, H.; Zhang, J.; Jiang, G. Bronsted acid catalyzed cyclization of inert N-substituted pyrroles to benzo[f]pyrrolo[1,2-a][1,4]diazepines. Synlett 2021, 32, 930–934. [Google Scholar] [CrossRef]
- Yata, T.; Nishimoto, Y.; Chiba, K.; Yasuda, M. Indium-catalyzed C-F bond transformation through oxymetalation/β-fluorine elimination to access fluorinated isocoumarins. Chem. Eur. J. 2021, 27, 8288–8294. [Google Scholar] [CrossRef]
- Marangoni, M.A.; Moraes, P.A.; Camargo, A.F.; Bonacorso, H.G.; Martins, M.A.P.; Zanatta, N. Synthesis of a novel 1,4-dicarbonyl scaffold-ethyl 3-formyl-4,5-dihydrofuran-2-carboxylate-and its application to the synthesis of pyridazines. Synthesis 2020, 52, 2528–2534. [Google Scholar] [CrossRef]
- Fedoseev, S.V.; Belikov, M.Y.; Ershov, O.V. Synthesis of 3-(dialkylamino)-4-halofuro[3,4-c]pyridin-1(3H)-ones. Rus. J. Org. Chem. 2020, 56, 49–52. [Google Scholar] [CrossRef]
- Kryshchyshyn-Dylevych, A.; Garazd, M.; Karkhut, A.; Polovkovych, S.; Lesyk, R. Synthesis and anticancer activity evaluation of 3-(4-oxo-2-thioxothiazolidin-5-yl)-1H-indole-carboxylic acids derivatives. Synth. Commun. 2020, 50, 2830–2838. [Google Scholar] [CrossRef]
- Wang, X.; Huang, D.; Wang, K.-H.; Su, Y.; Hu, Y. Tin powder-promoted cascade condensation/allylation/lactamization: Synthesis of isoindolinones and pyrazoloisoindol-8-ones. J. Org. Chem. 2019, 84, 6946–6961. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Xie, Z.; Lu, J.; Liu, J.; Cui, S.; Ma, Y.; Hu, X.; Liu, Y.; Zhong, K. Highly atom-economic, catalyst-free, and solvent-free synthesis of phthalazinones. ACS Sustain. Chem. Eng. 2019, 7, 134–138. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Matiychuk, V.S.; Obushak, M.D. Synthesis of isothiocoumarin derivatives. Chem. Heterocycl. Compd. 2010, 46, 140–145. [Google Scholar] [CrossRef]
- Kryshchyshyn-Dylevych, A.; Radko, L.; Finiuk, N.; Garazd, M.; Kashchak, N.; Posyniak, A.; Niemczuk, K.; Stoika, R.; Lesyk, R. Synthesis of novel indole-thiazolidinone hybrid structures as promising scaffold. Bioorg. Med. Chem. 2021, 50, 116453. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yu, N.; Chi, J.; Jia, M.; He, W.; He, F.; Tao, W.; Bai, C. Fused Imide Derivatives as Protein Degradation Targeted Chimera Inhibitors and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Diseases. Patent WO2021143816 22 July 2021. [Google Scholar]
- Wang, B.; Liu, J.; Tandon, I.; Wu, S.; Teng, P.; Liao, J.; Tang, W. Development of MDM2 degraders based on ligands derived from Ugi reactions: Lessons and Discoveries. Eur. J. Med. Chem. 2021, 219, 113425. [Google Scholar] [CrossRef]
- Morrow, B.J.; Hubert, J.G.; Dennis, M.L.; Cuzzupe, A.N.; Stupple, P.A. Benzothiophene, Thienopyridine and Thienopyrimidine Derivatives for the Modulation of STING and their Preparation. Patent WO2021009362 20 January 2021. [Google Scholar]
- Liu, T.; Sui, Z.; Ji, J. Preparation of thieno[3,2-b]pyrrole[3,2-d]pyridazinone Derivatives and Their Use as PKM2 Modulators for the Treatment of Cancer, Obesity and Diabetes-Related Disorders. Patent WO2020167976 12 February 2020. [Google Scholar]
- Jiang, X.-Q.; Chen, S.-Q.; Liu, Y.-F.; Pan, X.-G.; Chen, D.; Wang, S.-F. Solvothermal synthesis of multiple dihydropyrimidinones at a time as inhibitors of Eg5. Molecules 2021, 26, 1925. [Google Scholar] [CrossRef]
- Manjula, R.; Gokhale, N.; Unni, S.; Deshmukh, P.; Reddyrajula, R.; Srinivas Bharath, M.M.; Dalimba, U.; Padmanabhan, B. Design, synthesis, in-vitro evaluation and molecular docking studies of novel indole derivatives as inhibitors of SIRT1 and SIRT2. Bioorg. Chem. 2019, 92, 103281. [Google Scholar] [CrossRef]
- Guo, H.; Verhoek, I.C.; Prins, G.G.H.; van der Vlag, R.; van der Wouden, P.E.; van Merkerk, R.; Quax, W.J.; Olinga, P.; Hirsch, A.K.H.; Dekker, F.J. Novel 15-lipoxygenase-1 inhibitor protects macrophages from lipopolysaccharide-induced cytotoxicity. J. Med. Chem. 2019, 62, 4624–4637. [Google Scholar] [CrossRef] [PubMed]
- Cianchetta, G.; Liu, T.; Padyana, A.K.; Sui, Z.; Cai, Z.; Cui, D.; Ji, J. Thiasolopyrrolopyridazines as Pyruvate Kinase Activators for Use in Treating Blood Disorders and Their Preparation. Patent WO2019035863 21 February 2019. [Google Scholar]
- L’Abbe, G.; Dehaen, W. Synthesis and thermal rearrangement of 5-(diazomethyl)-1,2,3-triazoles. Tetrahedron 1988, 44, 461–469. [Google Scholar] [CrossRef]
- Storer, R.; Gosselin, G.; Dukhan, D.; Leroy, F. Preparation of Purine Nucleoside Analogs for Treating Flaviv Iridae Including Hepatitis C. Patent WO2005009418 3 February 2005. [Google Scholar]
- Kimura, T.; Tanaka, N.; Sugidachi, A.; Konosu, T. Preparation of Cyclic Amine Derivatives Having Heteroaryl Ring as Platelet Activation Inhibitors. Patent US20060270706 30 November 2006. [Google Scholar]
- Vroemans, R.; Horsten, T.; Van Espen, M.; Dehaen, W. 5-Formyltriazoles were used as valuable starting materials for unsymmetrically substituted bi-1,2,3-triazoles. Front. Chem. 2020, 8, 00271. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Shyyka, O.Y.; Matiychuk, V.S.; Obushak, M.D.; Pavlyuk, V.V. A novel base-solvent controlled chemoselective azide attack on an ester group versus keto in alkyl 3- substituted 3-oxopropanoates: Mechanistic insights. ChemistrySelect 2017, 2, 5871–5876. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Shyyka, O.Y.; Obushak, M.D. Convenient synthetic path to ethyl 1- aryl-5-formyl-1H-1,2,3-triazole-4-carboxylates and 1-aryl-1,5-dihydro-4H-[1,2,3]triazolo[4,5-d]pyridazin-4-ones. Chem. Heterocycl. Compd. 2018, 54, 773–779. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Shyyka, O.Y.; Savka, R.D.; Obushak, M.D. 2-Azido-1,3,4-thiadiazoles, 2-azido-1,3-thiazoles, and aryl azides in the synthesis of 1,2,3-triazole-4-carboxylic acids and their derivatives. Russ. J. Org. Chem. 2018, 54, 1090–1099. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Savka, R.D.; Pidlypnyi, N.I.; Matiychuk, V.S.; Obushak, M.D. Synthesis of 2-azido-1,3-thiazoles as 1,2,3-triazole precursors. Synth. Commun. 2010, 40, 391–399. [Google Scholar] [CrossRef]
- Kaushik, R.; Kushwaha, K.; Chand, M.; Vashist, M.; Jain, S.C. Design and synthesis of 2, 5-disubstituted-1, 3, 4-oxadiazole hybrids bearing pyridine and 1, 2, 3-triazole pharmacophores. J. Heterocycl. Chem. 2017, 54, 042–1047. [Google Scholar] [CrossRef]
- Pacifico, R.; Destro, D.; Gillick-Healy, M.W.; Kelly, B.G.; Adamo, M.F. Preparation of acidic 5-hydroxy-1, 2, 3-triazoles via the cycloaddition of aryl azides with β-ketoesters. J. Org. Chem. 2021, 86, 11354–11360. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.; Kesternich, V.; Pérez-Fehrmann, M.; Araneda, C.; Cárdenas, A. Crystal structure of ethyl 5-methyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate, C11H12N4O2. Zeitschrift für Krist.-New Cryst. Struct. 2017, 232, 1011–1012. [Google Scholar] [CrossRef]
- Piterskaya, Y.L.; Khramchikhin, A.V.; Stadnichuk, M.D. Cycloaddition of organic azides to α,β-acetylenic aldimines. Zh. Obshch. Khim. 1996, 66, 1187–1188. (In Russian) [Google Scholar]
- Pokhodylo, N.T.; Slyvk, Y.; Goreshnik, E.; Lytvyn, R. Synthesis, crystal structure and Hirshfeld surface analysis of (4-methylphenyl)[1-(pentafluorophenyl)-5-(trifluoromethyl)-1H-1,2,3-triazol-4-yl]methanone. Acta Crystallogr. E. 2021, 77, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Pokhodylo, N.T.; Slyvka, Y.; Pavlyuk, V. Synthesis, crystal structure and Hirshfeld surface analysis of 5-cyclopropyl-N-(2-hydroxyethyl)-1-(4-methylphenyl)-1H-1,2,3-Triazole-4-carboxamide. Acta Crystallogr. E. 2021, 77, 1043–1047. [Google Scholar] [CrossRef]
- Pokhodylo, N.; Slyvka, Y.; Pavlyuk, V. Synthesis, crystal structure and Hirshfeld surface analysis of N-(4-chlorophenyl)-5-cyclopropyl-1-(4-methoxyphenyl)-1H-1,2,3-triazole-4-carboxamide. Acta Crystallogr. E. 2020, 76, 756–760. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Shyyka, O.Y.; Goreshnik, E.A.; Obushak, M.D. 4-Phosphonated or 4-Free 1,2,3-Triazoles: What Controls the Dimroth Reaction of Arylazides with 2-Oxopropylphosphonates? ChemistrySelect 2020, 5, 260–264. [Google Scholar] [CrossRef]
- Bartlett, G.J.; Newberry, R.W.; VanVeller, B.; Raines, R.T.; Woolfson, D.N. Interplay of Hydrogen Bonds and n →π* Interactions in Proteins. J. Am. Chem. Soc. 2013, 135, 18682–18688. [Google Scholar] [CrossRef] [Green Version]
- Newberry, R.W.; Raines, R.T. The n →π* Interaction. Acc. Chem. Res. 2017, 50, 1838–1846. [Google Scholar] [CrossRef] [Green Version]
- Gorske, B.C.; Bastian, B.L.; Geske, G.D.; Blackwell, H.E. Local and Tunable n→π* Interactions Regulate Amide Isomerism in the Peptoid Backbone. J. Am. Chem. Soc. 2007, 129, 8928–8929. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Choudhury, S.R.; Estarellas, C.; Dey, B.; Frontera, A.; Hemming, J.; Helliwell, M.; Gamez, P.; Mukhopadhyay, S. Supramolecular assemblies involving anion–π and lone pair–π interactions: Experimental observation and theoretical analysis. CrystEngComm 2011, 13, 4519. [Google Scholar] [CrossRef]
- Bondi, A.V. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.J.; Mckinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17. The University of Western Australia. 2017. Available online: http://hirshfeldsurface.net (accessed on 3 February 2022).
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Turner, M.J.; Thomas, S.P.; Shi, M.W.; Jayatilaka, D.; Spackman, M.A. Energy frameworks: Insights into interaction anisotropy and the mechanical properties of molecular crystals. Chem. Commun. 2015, 51, 3735–3738. [Google Scholar] [CrossRef]
- Priebbenow, D.L.; Zou, L.H.; Becker, P.; Bolm, C. The Disubstitution of Acetals to Prepare δ,δ-Bis (aryl) β-Keto Esters. Eur. J. Org. Chem. 2013, 2013, 3965–3969. [Google Scholar] [CrossRef]
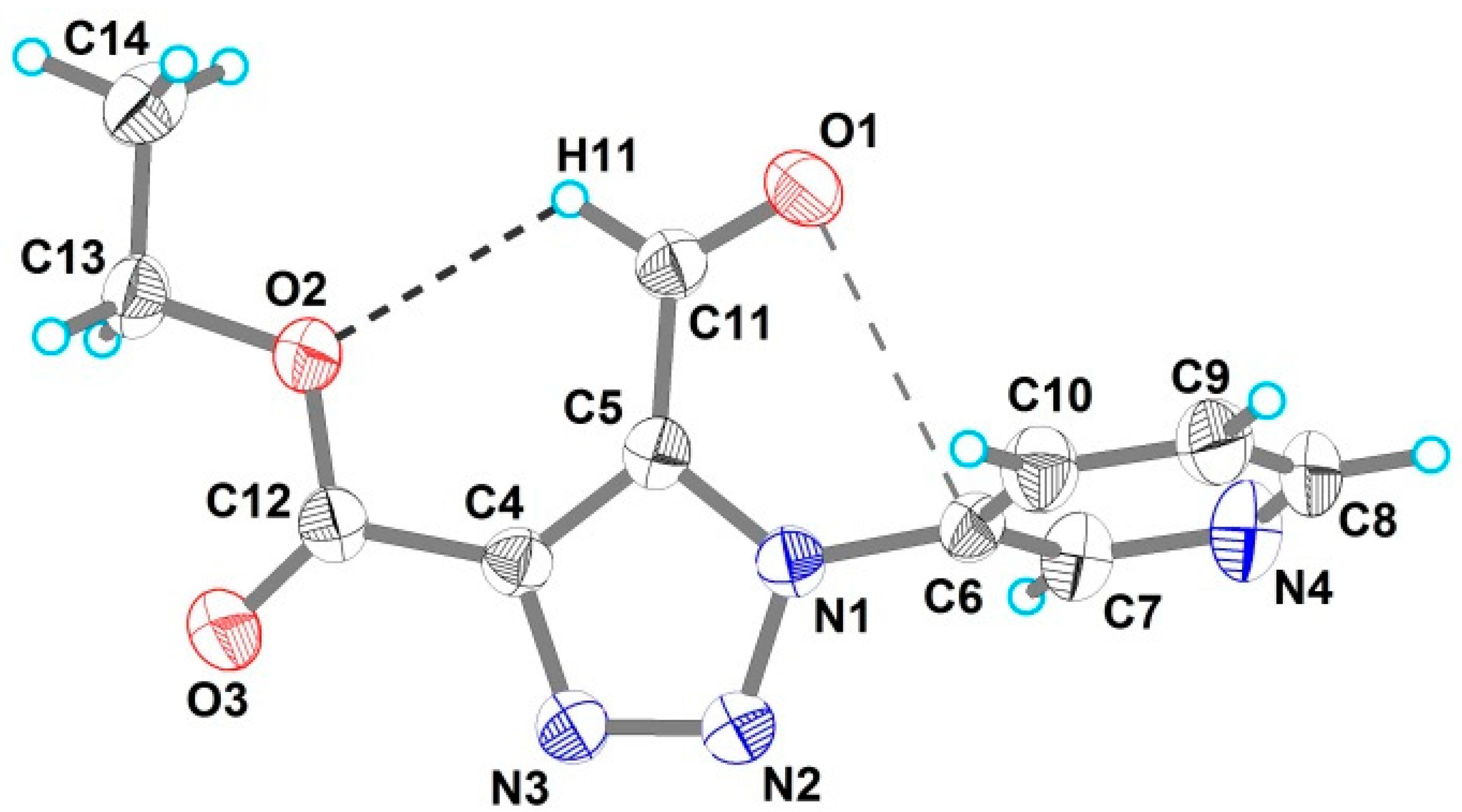
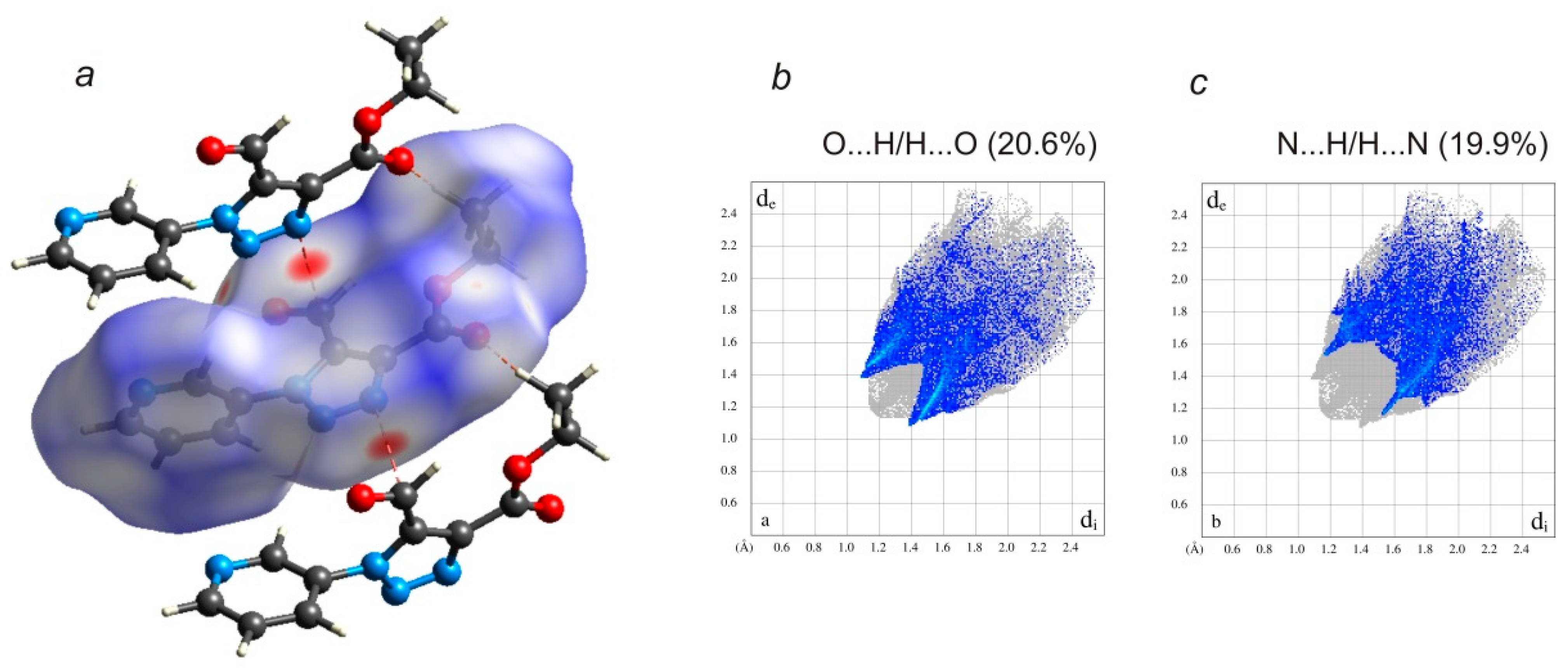

| Bond | Å | Angles | ° |
|---|---|---|---|
| C11–O11 | 1.199(3) | N1–C5–C11 | 124.5(2) |
| C5–C11 | 1.478(4) | O1–C11–C5 | 123.7(3) |
| N1–C6 | 1.444(3) | O2–C12–C4 | 125.0(3) |
| N1–N2 | 1.358(3) | N1–C5–C11–O1 | −3.5(4) |
| C12–O3 | 1.191(3) | C5–C4–C12–O2 | −3.3(4) |
| Atoms Involved | Symmetry | Distances, Å | Angle, Deg | ||
|---|---|---|---|---|---|
| D–H···A | D–H | H···A | D···A | D–H···A | |
| C10–H10···O1 | x, −y + 2, z + 0.5 | 0.95 | 2.55 | 3.267(4) | 132 |
| C10–H10···N2 | x, y + 1, z | 0.95 | 2.79 | 3.562(4) | 139 |
| C14–H14B···O3 | x, y + 1, z | 0.98 | 2.67 | 3.592(4) | 157 |
| C8–H8···O3 | x + 0.5, y + 0.5 | 0.95 | 2.69 | 3.617(4) | 164 |
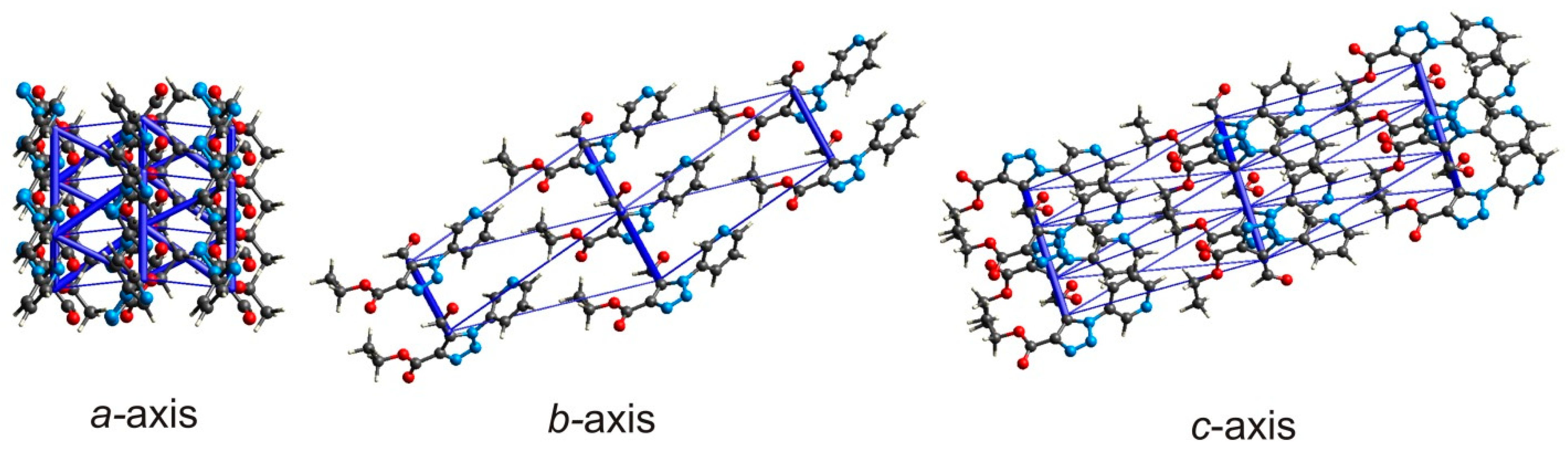
| No a | Symmetry Codes | R b | E_ele | E_pol | E_dis | E_rep | E_tot c |
|---|---|---|---|---|---|---|---|
| MI | (i) x, y + 1, z (vi) x, y − 1, z | 5.35 | −18.7 | −4.8 | −35.2 | 24.1 | −39.1 |
| MII | (v) x, −y + 1, z + 0.5 (viii) x, −y + 1, z − 0.5 | 5.11 | −17.9 | −7.1 | −32.5 | 21.8 | −39.1 |
| MIII | (ix) x + 0.5, −y + 1.5, z + 0.5 (x) x − 0.5, −y + 1.5, z − 0.5 | 12.25 | −1.7 | −0.9 | −10.7 | 6.8 | −7.6 |
| MIV | (ii) x, −y + 2, z + 0.5 (vii) x, −y + 2, z − 0.5 | 5.31 | −10.1 | −3.8 | −35.9 | 23.5 | −30.3 |
| MV | (iii) x + 0.5, y + 0.5, z; (iv) x − 0.5, y − 0.5, z | 12.19 | −3.9 | −1.7 | −7.9 | 6.0 | −8.6 |
| MVI | (xi) x + 0.5, y − 0.5, z (xii) x − 0.5, y + 0.5, z | 12.19 | −4.2 | −1.6 | −9.0 | 5.7 | −10.0 |
| MVII | (xiii) x + 0.5, −y + 2.5, z + 0.5 (xiv) x − 0.5, −y + 2.5, z − 0.5 | 13.44 | 1.9 | −0.5 | −3.9 | 2.6 | −0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhodylo, N.T.; Slyvka, Y.I.; Goreshnik, E.A.; Obushak, M.D. Ethyl 5-Formyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and DFT Calculation. Molbank 2022, 2022, M1340. https://doi.org/10.3390/M1340
Pokhodylo NT, Slyvka YI, Goreshnik EA, Obushak MD. Ethyl 5-Formyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and DFT Calculation. Molbank. 2022; 2022(1):M1340. https://doi.org/10.3390/M1340
Chicago/Turabian StylePokhodylo, Nazariy T., Yuriy I. Slyvka, Evgeny A. Goreshnik, and Mykola D. Obushak. 2022. "Ethyl 5-Formyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and DFT Calculation" Molbank 2022, no. 1: M1340. https://doi.org/10.3390/M1340
APA StylePokhodylo, N. T., Slyvka, Y. I., Goreshnik, E. A., & Obushak, M. D. (2022). Ethyl 5-Formyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and DFT Calculation. Molbank, 2022(1), M1340. https://doi.org/10.3390/M1340







