Abstract
The pharmacophore hybridization approach is widely used for the design of drug-like small molecules with anticancer properties. In the present work, a “cost-effective” approach to the synthesis of the novel non-condensed pyrazoline-bearing hybrid molecule with 1,3,4-thiadiazole and dichloroacetic acid moieties is proposed. The 5-amino-1,3,4-thiadiazole-2-thiol was used as a starting reagent, and the synthetic strategy includes stepwise alkylation of the sulfur atom and acylation of the nitrogen atom to obtain the target title compound. The structure of the synthesized 2,2-dichloro-N-[5-[2-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydro-2H-pyrazol-2-yl]-2-oxoethyl]sulfanyl-1,3,4-thiadiazol-2-yl]acetamide (yield 90%) was confirmed by 1H, 13C, 2D NMR and LC-MS spectra. Anticancer activity in “60 lines screening” (NCI DTP protocol) was studied in vitro for the title compound.
1. Introduction
The pharmacophore hybridization approach plays a key and leading role in the modern medicinal chemistry of anticancer agents [1,2]. The application of the hybridization concept allows the design and discovery of new small molecules as high-affinity ligands to potential anticancer targets, as well as fighting and overcoming the multidrug resistance problems [3,4,5]. The synthetic pathways applied for the obtaining of new anticancer agents in the molecular hybridization context require fast, easy, and cheap (“cost-effective”) synthetic schemes to the target hybrid molecules with their high yield and purity [6,7,8].
The small molecules containing substituted pyrazoline and 1,3,4-thiadiazole scaffolds are the leading objects in the modern anticancer agent drug design and present the important groups of anticancer drugs (Figure 1) [9,10,11]. Pyrazoline-based molecules are available in the pharmaceutical market as approved drugs targeted at different types of cancer diseases: crizotinib—inhibitor of the receptor tyrosine kinases c-MET, ALK and ROS1 [12,13,14]; ruxolitinib—JAK 1 and 2 inhibitor [15,16]; encorafenib—selective BRAF inhibitor (BRAFi) [17]; darolutamide—androgen receptor (AR) antagonist [18]. 1,3,4-Thiadiazole derivatives demonstrate in vitro and/or in vivo efficacy across the cancer models [19,20] and are currently at different stages of clinical trials as prospective inhibitors of carbonic anhydrase [21], glutaminase [22], inosine monophosphate dehydrogenase [23], and kinesin spindle protein (KSP, Eg5) [24]. In the last two decades, the number of reports describing and supporting dichloroacetic acid (DCA) employment against some cancer types has increased [25]. Currently, DCA and some bioisosteres are intensively studied and evaluated in the synergistic conditions/experiments with other anticancer agents of synthetic and natural origin for the optimization of anticancer potency [25,26].
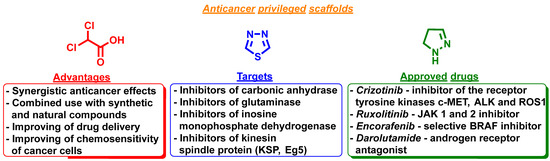
Figure 1.
Dichloroacetic acid, 1,3,4-thiadiazole, and pyrazoline scaffolds in modern anticancer drug designs.
In this regard, the main object of the present work was developing a fast and easy synthetic scheme for hybrid molecules containing pyrazoline, 1,2,4-thiadiazole, and dichloroacetic acid moieties, using cheap, commercially available reagents. The structure characterization of the synthesized molecule, using NMR and LC-MS spectra and in vitro anticancer activity evaluation accordingly to the “60 lines screening” algorithm (DTP NCI, USA), are presented herein as well.
2. Results and Discussion
2.1. Synthesis of the Title Compound 3
For the synthesis of the title compound 3, the following “cost-effective” approach (Scheme 1) was proposed. The 5-amino-1,3,4-thiadiazole-2-thiol (1), which could be easily synthesized or be cheaply purchased, was used as a starting reagent. Initially, potassium 5-amino-1,3,4-thiadiazole-2-thiolate was generated in situ by the soft and short heating of 1 with an ethanolic solution of potassium hydroxide. Then, 2-chloro-1-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydropyrazol-2-yl]ethanone and catalytic amount (0.1 mmol%) of potassium iodide was added and the reaction mixture refluxed for 1.5 h. The selective alkylation of the exocyclic sulfur atom in 1 was achieved using such an approach, and intermediate compound 2 was obtained with an 81% yield. Differences in the nucleophilic properties of thiol- and amine-group in 1 were the main reason for selective alkylation, and such an approach is popular in the design of bioactive 1,3,4-thiadiazoles [27,28].

Scheme 1.
Synthesis of title compound 3. Reagents and conditions: (i)—1 (10 mmol), KOH (10 mmol), 2-chloro-1-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydro-2H-pyrazol-2-yl]ethanone (11 mmol), KI (0.1 mmol%), ethanol (20 mL), reflux 1.5 h; (ii)—2 (10 mmol), triethylamine (10 mmol), dichloroacetyl chloride (10 mmol), dioxane (15 mL), reflux 30 min.
In the next step, the acylation of the primary amino group of 2 was applied, and the commercially available dichloroacetyl chloride was used as an acylating agent. The reaction was performed in the dry dioxane in the presence of triethylamine by refluxing for 30 min, and target compound 3 was obtained with a yield of 90%.
The structure of synthesized compound 3 was confirmed using 1H, 13C NMR, and LC-MS spectra (copies of spectra are presented in the supplementary materials). In the 1H NMR spectrum of compound 3, the characteristic signals of all protons are presented. The proton of the amine-group resonated as a broad singlet at 13.57 ppm. The aromatic protons were characterized by three doublets at 6.87, 7.13, 7.80 ppm, and broad signal at 7.48 ppm. The signal of the proton in the dichloroacetic acid residue shifted in the weak field and resonated as a strong singlet at 6.68 ppm. The protons of the pyrazoline moiety had a pattern with three doublet of doublets at 5.54, 3.88, and 3.17 ppm, with appropriate spin–spin coupling constants. The two protons of the methylene group were magnetic non-equivalent and gave two doublets at 4.55 and 4.69 ppm with J = 15.6 Hz. The methyl group protons resonated as a singlet at 3.71 ppm. The assignment of protons signals was confirmed using 1H COSY technique.
The 13C NMR spectra of 3 showed 15 carbon signals, and some signals were overlapping. Additional application of correlation 2D NMR technics (HSQC and HMBC) allowed to distinguish that in the 13C NMR spectra of 3 two signals of aliphatic, and nine aromatic methines, two methylene, and one methyl carbons were presented, as well one carbonyl carbon. Carbons of aromatic methines resonated in the range from 114.5 to 159.0 ppm and overlapped with each other, as well with the carbonyl group carbon (C-30) signal. The carbon atom of the carbonyl group at C-3 resonated at 164.5 ppm.
In the IR-spectra of 2 and 3, the absorption bands at 1696 and 1645 cm−1 are assigned to the C=O stretching vibrations, respectively. The absorption bands at 3312, 3120 cm−1 appeared in the IR-spectrum of 3 and corresponded to the NH vibrations.
The molecular ion peak observed at an m/z value of 538.0 [M + H]+ in the positive ionization mode in the mass spectrum confirmed the formation of the title compound 3.
2.2. In Vitro Evaluation of the Anticancer Activity of Compound 3
The antitumor activity screening was performed for title compound 3, according to the standard protocols of the National Cancer Institute (NCI, Bethesda, MD, USA) Developmental Therapeutic Program (DTP) [29,30,31,32]. The screening process included evaluation of antitumor activity at the concentration of 10 µM against a panel of approximately sixty cancer cell lines representing different types of cancer, including leukemia, melanoma, lung, colon, CNS, ovarian, renal, prostate, and breast cancers. The results of the screening assay are summarized in Table 1, and the complete data are presented in the supplementary materials.

Table 1.
Anticancer activity data of compound 3 at concentration 10 μM.
The screening results revealed that synthesized derivative 3 possessed a low level of anticancer activity and only four cancer cell lines (Table 1) were slightly sensitive at 10 μM. In general, it should be noted that the leukemia lines were most sensitive to the impact of compound 3.
3. Materials and Methods
Melting points were measured in open capillary tubes on a BÜCHI B-545 melting point apparatus (BÜCHI Labortechnik AG, Flawil, Switzerland), and were uncorrected. The elemental analyses (C, H, N) were performed using the Perkin-Elmer 2400 CHN analyzer (PerkinElmer, Waltham, MA, USA) and were within ±0.4% of the theoretical values. The 400 MHz 1H and 126 MHz 13C NMR spectra were recorded on Varian Unity Plus 400 (400 MHz) spectrometer (Varian Inc., Paulo Alto, CA, USA). All spectra were recorded at room temperature, except where indicated otherwise, and were referenced internally to solvent reference frequencies. Chemical shifts (δ) are quoted in ppm and coupling constants (J) are reported in Hz. LC-MS spectra were obtained on a Finnigan MAT INCOS-50 (Thermo Finnigan LLC, San Jose, CA, USA). The reaction mixture was monitored by thin layer chromatography (TLC) using commercial glass-backed TLC plates (Merck Kieselgel 60 F254). Solvents and reagents that are commercially available were used without further purification. Starting compound 1 was prepared according to protocol described in [33]. The 2-chloro-1-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydropyrazol-2-yl]ethanone was accomplished by the reaction of 2-chloroacetyl chloride with appropriate 5-(4-methoxyphenyl)-3-phenyl-4,5-dihydro-1H-pyrazole according to the reported method described in [7].
2-[(5-Amino-1,3,4-thiadiazol-2-yl)sulfanyl]-1-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydro-2H-pyrazol-2-yl]ethanone (2)
To a stirred solution of 2-amino-5-mercapto-1,3,4-thiadiazole (1) (1.33 g, 10 mmol) in ethanol (10 mL) at ca. 20 °C, was added a potassium hydroxide (0.56 g, 10 mmol) solution in ethanol (10 mL). The mixture was then heated to reflux for 15 min. Then, the 2-chloro-1-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydropyrazol-2-yl]ethanone (3.62 g, 11 mmol) and potassium iodide (1.66 mg, 10 µmol) were added to the reaction mixture and the mixture was then heated to reflux for 1.5 h (monitored by TLC). After completion, the reaction mixture was cooled to the room temperature. The resultant white solid of compound 2 was collected by filtration, washed with methanol (5–10 mL), diethyl ether and crystallized from the mixture DMF:ethanol (1:2).
Yield 81%, white powder, mp 209–211 °C (DMF:EtOH (1:2)). 1H NMR (400 MHz, DMSO-d6, δ): 3.15 (dd, 1H, J = 4.5, 18.1 Hz, CH2), 3.72 (s, 3H, OCH3), 3.86 (dd, 1H, J = 11.8, 18.1 Hz, CH2), 4.32 (d, 1H, J = 15.1 Hz, CH2), 4.45 (d, 1H, J = 15.1 Hz, CH2), 5.52 (dd, 1H, J = 4.4, 11.6 Hz, CH), 6.87 (d, 2H, J = 8.6 Hz, arom.), 7.14 (d, 2H, J = 8.6 Hz, arom.), 7.29 (s, 2H, NH2), 7.44–7.50 (m, 3H, arom.), 7.80 (d, 2H, J = 7.5 Hz, arom.).
13C NMR (126 MHz, DMSO-d6, δ): 37.6 (CH2), 42.6 (CH2), 55.6 (CH3), 59.8 (CHal.), 114.4 (CHar.), 127.3 (CHar.), 129.2 (CHar.), 129.3 (CHar.), 131.0 (CHar.), 131.2 (CHar.), 134.2 (CHar.), 149.9 (CHar.), 155.7 (CHar.), 158.9 (CHar.), 164.9 (CHar.), 170.4 (C=O).
IR (KBr): 1696, 1657 (C=O), 1563 (C=C) cm−1.
LCMS (Electrospray ionization (ESI+)): m/z 426.2 (100%, [M + H]+)
Anal. calc. for C20H19N5O2S2: C 56.45%, H 4.50%, N 16.46%. Found: C 56.62%, H 4.63%, N 16.58%.
2,2-Dichloro-N-[5-[2-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydro-2H-pyrazol-2-yl]-2-oxoethyl]sulfanyl-1,3,4-thiadiazol-2-yl]acetamide (3)
A mixture of compound 2 (2.12 g, 5 mmol) and triethylamine (0.5 g, 5 mmol) in dry dioxane (10 mL) was slightly heated to complete dissolving. Then, dichloroacetyl chloride (0.74 g, 5 mmol) in dry dioxane (5 mL) was added slowly to the reaction mixture during intensive stirring, and the mixture was then heated to reflux for 30 min (monitored by TLC). After completion, the reaction mixture was cooled to room temperature. The resultant white solid of compound 3 was collected by filtration, washed with petroleum ether (5–10 mL), and crystallized from the toluene.
Yield 90%, white powder, mp 140–142 °C (Toluene). 1H NMR (400 MHz, DMSO-d6, δ): 3.17 (dd, 1H, J = 4.1, 18.2 Hz, CH2), 3.71 (s, 3H, OCH3), 3.88 (dd, 1H, J = 11.7, 18.2 Hz, CH2), 4.55 (d, 1H, J = 15.6 Hz, CH2), 4.69 (d, 1H, J = 15.6 Hz, CH2), 5.54 (dd, 1H, J = 4.0, 11.3 Hz, CH2), 6.68 (s, 1H, CH), 6.87 (d, 2H, J = 8.2 Hz, arom.), 7.13 (d, 2H, J = 8.2 Hz, arom.), 7.48 (br.s, 3H, arom.), 7.80 (d, 2H, J = 7.0 Hz), 13.57 (br.s, 1H, NH).
13C NMR (126 MHz, DMSO-d6, δ): 37.2 (CH2, C-2), 42.7 (CH2, C-8), 55.6 (CH3, C-23), 60.0 (CHal., C-9), 66.6 (CHal., C-32), 114.5 and 127.3 and 129.3 (8*CHar., C-12,13; C-15,16; C-17,18; C-20,21), 128.7 (CHar., C-14), 131.1 (CHar., C-11), 134.1 and 134.2 (CHar.,C-10), 156.0 (2*CHar., C-7 and C-27), 159.0 (2*CHar.+ C=O, C-19 and C-24 and C-30), 164.5 (C=O, C-3).
IR (KBr): 3312, 3120 (NH), 1645, 1598 (C=O), 1517 (C=C) cm−1.
LCMS (Electrospray ionization (ESI+)): m/z 538.0/540.0 (100.0%, [M + H]+)
Anal. calc. for C22H19Cl2N5O3S2: C 49.26%, H 3.57%, N 13.05%. Found: C 49.39%, H 3.72%, N 13.18%.
4. Conclusions
In the present work, the “cost-effective” synthetic approach to novel non-condensed hybrid molecules containing pyrazoline, 1,3,4-thiadiazole, and dichloroacetic acid moieties was proposed. The target title compound—2,2-dichloro-N-[5-[2-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydro-2H-pyrazol-2-yl]-2-oxoethyl]sulfanyl-1,3,4-thiadiazol-2-yl]acetamide was synthesized using the proposed approach, and its structure was confirmed by 1H, 13C NMR, and LC-MS spectra. The in vitro anticancer activity of the title compound was studied, and low cytotoxicity effects on cell lines of leukemia (K-562, SR), colon cancer (HCT-15), and melanoma (SK-MEL-5) were identified. The obtained results contribute to the medicinal chemistry of heterocyclic hybrid molecules as possible anticancer agents.
Supplementary Materials
The following are available online. Figures S1–S7: 1H NMR, 13C NMR and LC–MS spectra of compounds 2 and 3; data of anticancer activity of compound 3.
Author Contributions
Conceptualization, I.Y., S.H. and R.L.; methodology, I.Y., S.H. and R.L.; software, I.Y., S.H.; validation, I.Y., S.H.; investigation, I.Y., S.H.; writing—original draft preparation, I.Y., S.H. and R.L.; writing—review and editing, I.Y., S.H. and R.L.; supervision, R.L.; project administration, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from Ministry of Healthcare of Ukraine (0121U100690), and the National Research Foundation of Ukraine (2020.02/0035).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
This research was supported by the Danylo Halytsky Lviv National Medical University which is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ortega, J.A.; Riccardi, L.; Minniti, E.; Borgogno, M.; Arencibia, J.M.; Greco, M.L.; Minarini, A.; Sissi, C.; De Vivo, M. Pharmacophore hybridization to discover novel topoisomerase II poisons with promising antiproliferative activity. J. Med. Chem. 2018, 61, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Okuhira, K.; Demizu, Y.; Hattori, T.; Ohoka, N.; Shibata, N.; Nishimaki-Mogami, T.; Okuda, H.; Kurihara, M.; Naito, M. Development of hybrid small molecules that induce degradation of estrogen receptor-alpha and necrotic cell death in breast cancer cells. Cancer Sci. 2013, 104, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef]
- Dothager, R.S.; Putt, K.S.; Allen, B.J.; Leslie, B.J.; Nesterenko, V.; Hergenrother, P.J. Synthesis and identification of small molecules that potently induce apoptosis in melanoma cells through G1 cell cycle arrest. J. Am. Chem. Soc. 2005, 127, 8686–8696. [Google Scholar] [CrossRef] [PubMed]
- Matiadis, D.; Mavroidi, B.; Panagiotopoulou, A.; Methenitis, C.; Pelecanou, M.; Sagnou, M. (E)-(1-(4-Ethoxycarbonylphenyl)-5-(3, 4-dimethoxyphenyl)-3-(3, 4-dimethoxystyryl)-2-pyrazoline: Synthesis, Characterization, DNA-Interaction, and Evaluation of Activity against Drug-Resistant Cell Lines. Molbank 2020, 2020, M1114. [Google Scholar] [CrossRef] [Green Version]
- Holota, S.; Komykhov, S.; Sysak, S.; Gzella, A.; Cherkas, A.; Lesyk, R. Synthesis, Characterization and In Vitro Evaluation of Novel 5-Ene-thiazolo [3,2-b][1,2,4] triazole-6 (5H)-ones as Possible Anticancer Agents. Molecules 2021, 26, 1162. [Google Scholar] [CrossRef] [PubMed]
- Holota, S.; Yushyn, I.; Khyluk, D.; Vynnytska, R.; Lesyk, R. N-(3-Cyano-4,5,6,7-tetrahydrobenzothiophen-2-yl)-2-[[5-[(1, 5-dimethyl-3-oxo-2-phenylpyrazol-4-yl) amino]-1,3,4-thiadiazol-2-yl] sulfanyl] acetamide. Molbank 2021, 2021, M1211. [Google Scholar] [CrossRef]
- Tok, F.; Abas, B.İ.; Çevik, Ö.; Koçyiğit-Kaymakçıoğlu, B. Design, synthesis and biological evaluation of some new 2-Pyrazoline derivatives as potential anticancer agents. Bioorg. Chem. 2020, 102, 104063. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Holota, S.; Yushyn, I.; Sabadakh, O.; Karpenko, O.; Novikov, V.; Lesyk, R. Synthesis and Biological Activity Evaluation of Polyfunctionalized Anthraquinonehydrazones. Lett. Drug Des. Discov. 2021, 18, 199–209. [Google Scholar] [CrossRef]
- Edrees, M.M.; Melha, S.A.; Saad, A.M.; Kheder, N.A.; Gomha, S.M.; Muhammad, Z.A. Eco-friendly synthesis, characterization and biological evaluation of some novel pyrazolines containing thiazole moiety as potential anticancer and antimicrobial agents. Molecules 2018, 23, 2970. [Google Scholar] [CrossRef] [Green Version]
- Altıntop, M.D.; Sever, B.; Özdemir, A.; Ilgın, S.; Atlı, Ö.; Turan-Zitouni, G.; Kaplancıklı, Z.A. Synthesis and evaluation of a series of 1,3,4-thiadiazole derivatives as potential anticancer agents. Anti-Cancer Agents Med. Chem. 2018, 18, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Blackhall, F.; Cappuzzo, F. Crizotinib: From discovery to accelerated development to front-line treatment. Ann. Oncol. 2016, 27, iii35–iii41. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Prabhash, K.; Noronha, V.; Joshi, A.; Desai, S. Crizotinib: A comprehensive review. South Asian J. Cancer 2013, 2, 91. [Google Scholar]
- Ou, S.H.I.; Bartlett, C.H.; Mino-Kenudson, M.; Cui, J.; Iafrate, A.J. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: A success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist 2012, 17, 1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.P.; Keating, G.M. Ruxolitinib. Drugs 2012, 72, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.; Kiladjian, J.J.; Al-Ali, H.K.; Gisslinger, H.; Waltzman, R.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.; Knoops, L.; et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 2012, 366, 787–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Zurth, C.; Koskinen, M.; Fricke, R.; Prien, O.; Korjamo, T.; Graudenz, K.; Denner, K.; Bairlein, M.; Von Buehler, C.J.; Wilkinson, G.; et al. Drug–drug interaction potential of darolutamide: In vitro and clinical studies. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 747–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliabadi, A. 1,3,4-Thiadiazole based anticancer agents. Anti-Cancer Agents Med. Chem. 2016, 16, 1301–1314. [Google Scholar] [CrossRef]
- Szeliga, M. Thiadiazole derivatives as anticancer agents. Pharmacol. Rep. 2020, 72, 1–22. [Google Scholar] [CrossRef]
- Askin, S.; Tahtaci, H.; Türkeş, C.; Demir, Y.; Ece, A.; Çiftçi, G.A.; Beydemir, Ş. Design, synthesis, characterization, in vitro and in silico evaluation of novel imidazo[2,1-b][1,3,4]thiadiazoles as highly potent acetylcholinesterase and non-classical carbonic anhydrase inhibitors. Bioorg. Chem. 2021, 113, 105009. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Malik, D.; Perkons, N.; Huangyang, P.; Khare, S.; Rhoades, S.; Gong, Y.Y.; Burrows, M.; Finan, J.M.; Nissim, I.; et al. Targeting glutamine metabolism slows soft tissue sarcoma growth. Nat. Commun. 2020, 11, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Monte, C.; Carradori, S.; Secci, D.; D’Ascenzio, M.; Guglielmi, P.; Mollica, A.; Morrone, S.; Scarpa, S.; Aglianò, A.M.; Giantulli, S.; et al. Synthesis and pharmacological screening of a large library of 1,3,4-thiadiazolines as innovative therapeutic tools for the treatment of prostate cancer and melanoma. Eur. J. Med. Chem. 2015, 105, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Patnaik, A.; Verschraegen, C.F.; Olszanski, A.J.; Shaheen, M.; Burris, H.A.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Hynes, S.M.; et al. Two Phase 1 dose-escalation studies exploring multiple regimens of litronesib (LY2523355), an Eg5 inhibitor, in patients with advanced cancer. Cancer Chemother. Pharmacol. 2017, 79, 315–326. [Google Scholar] [CrossRef]
- Tataranni, T.; Piccoli, C. Dichloroacetate (DCA) and cancer: An overview towards clinical applications. Oxid. Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Serrano-Sterling, C.; Becerra, D.; Portilla, J.; Rojas, H.; Macías, M.; Castillo, J.-C. Synthesis, biological evaluation and X-ray crystallographic analysis of novel (E)-2-cyano-3-(het)arylacrylamides as potential anticancer agents. J. Mol. Struct. 2021, 1244, 130944. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Ramadan, E.S.; Amer, M.R.; El Kilany, Y.; Badawy, M.E.I.; Rabea, E.I. Synthesis and Antioxidant Activity of Novel 5-amino-2-alkyl/glycosylthio-1,3,4-thiadiazoles: Regioselective Alkylation and Glycosylation of the 5-amino-1,3,4- thiadiazole-2-thiol Scaffold. Curr. Org. Synth. 2019, 16, 801–809. [Google Scholar] [CrossRef]
- Drapak, I.V.; Zimenkovsky, B.S.; Slabyy, M.V.; Holota, S.M.; Perekhoda, L.O.; Yaremkevych, R.V.; Nektegayev, I.O. Synthesis and diuretic activity of novel 5-amino-1,3,4-thiadiazole-2-thiol derivatives. Biopolym. Cell 2021, 37, 33–45. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Boyd, M.R. The NCI In Vitro Anticancer Drug Discovery Screen. In Anticancer Drug Development Guide; Teicher, B.A., Ed.; Humana Press: Totowa, NJ, USA, 1997; Volume 2, pp. 23–42. [Google Scholar]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Al-Shibani, I.S.; Al-Yonis, N.K.; Al-Dujaili, A.H. Synthesis and characterization of some new 1,3,4-thiadiazol derivatives. Irq. Nat. J. Chem. 2006, 21, 94–101. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).