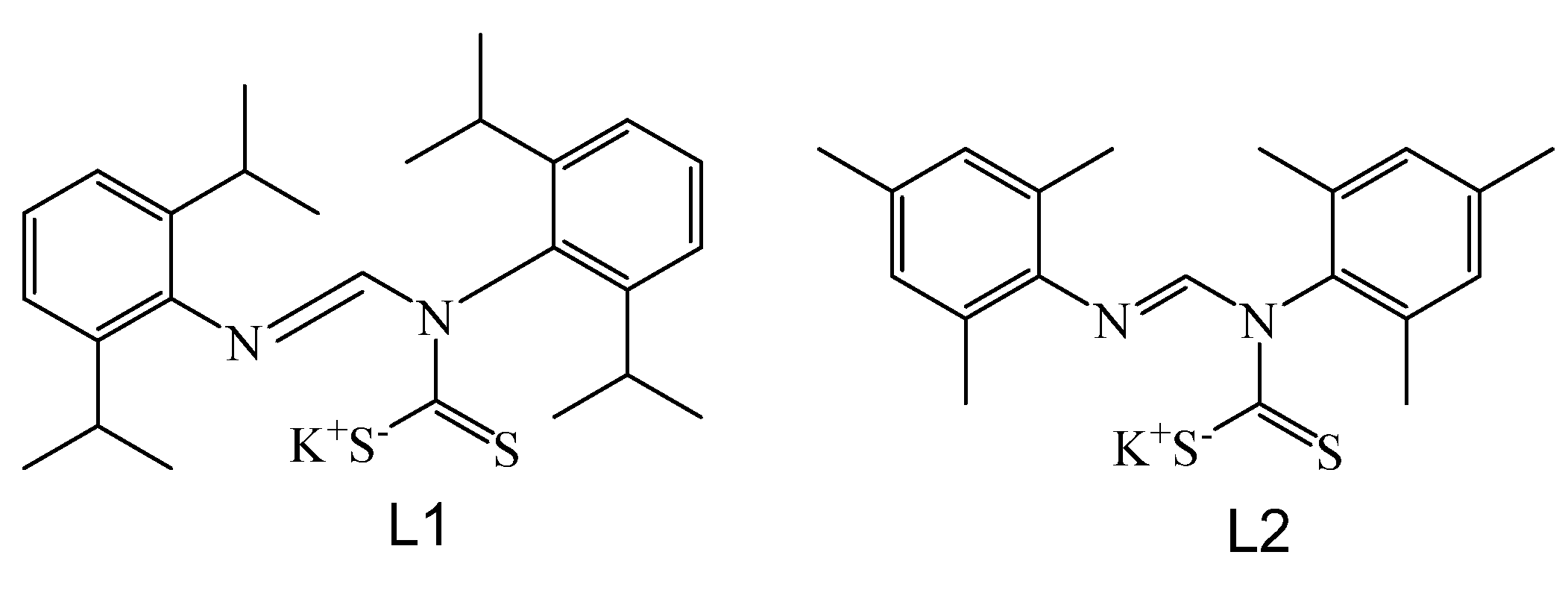
N,N′-Diarylformamidine Dithiocarbamate Ag(I) Cluster and Coordination Polymer
Abstract
:1. Introduction
2. Materials and Instrumentation
3. General Synthesis Methods
3.1. Preparation of Complexes
3.1.1. Ag6(L1)6 1
3.1.2. Ag2(L2)2 2
3.2. Single Crystal X-ray Diffraction
4. Results and Discussion
4.1. Synthesis of N,N′-Diarylformamidines Dithiocarbamate Ag(I) Complexes
4.2. Spectroscopic Studies
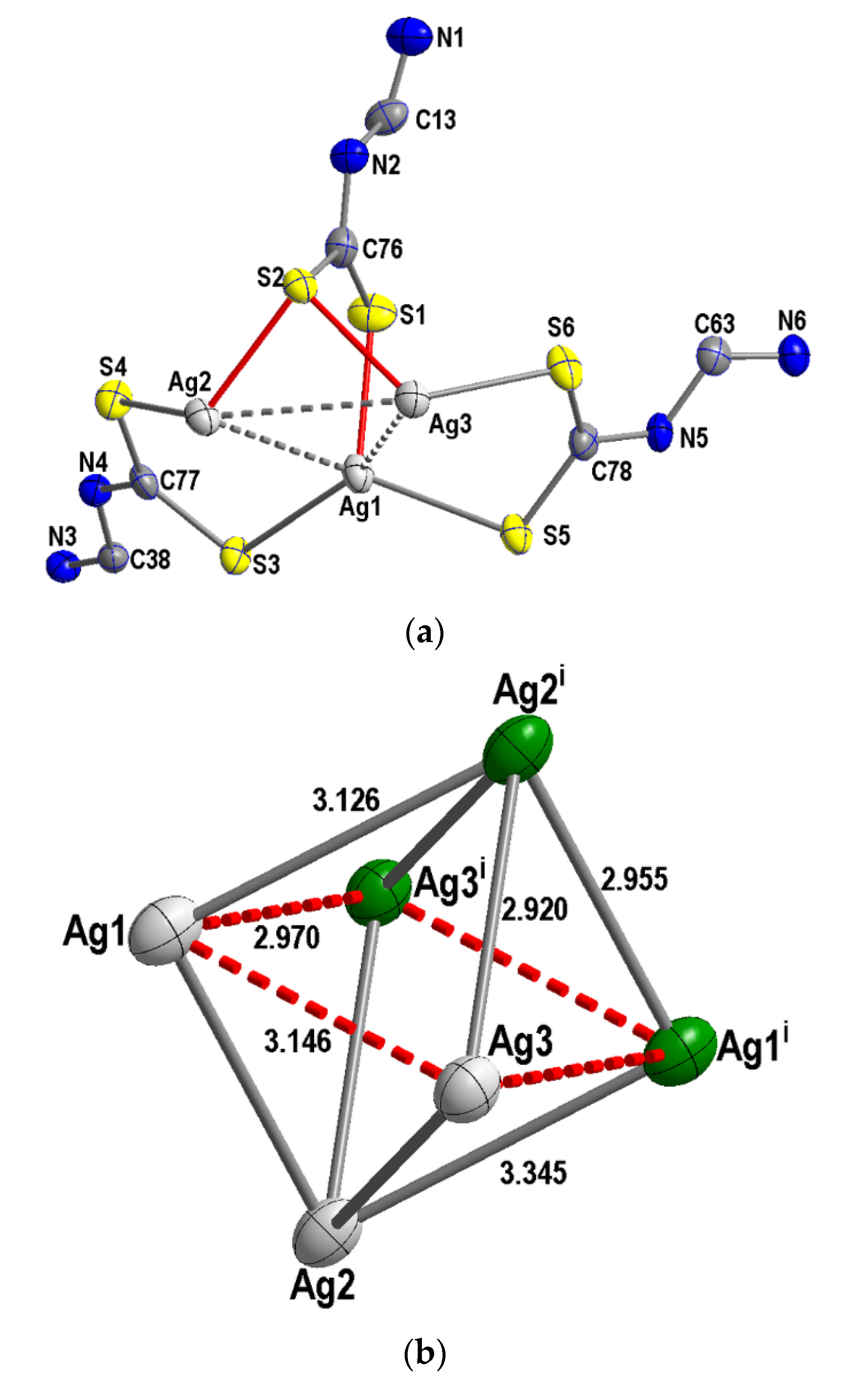
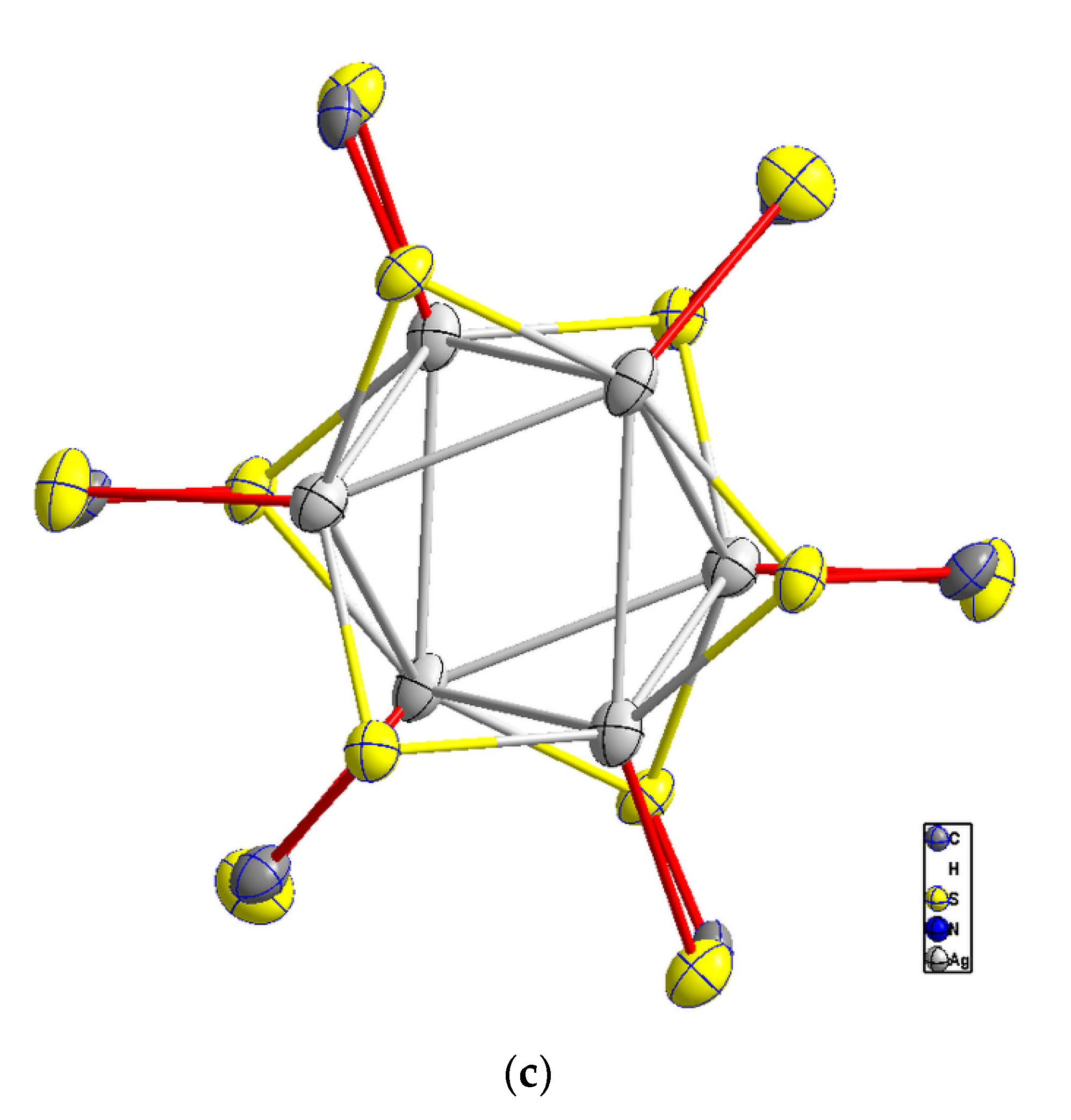
4.3. X-ray Structural Analysis
Comparison of 1 to Ag6[CS2(2,6- iPr2C6H3NC(H)=NC6H3- iPr2)]6
4.4. Thermal Decomposition Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, A.G.; Hanton, L.R. Square planar silver(I) complexes: A rare but increasing observed stereochemistry for silver(I). Coord. Chem. Rev. 2008, 252, 1346–1386. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Argentophilic interactions. Angew. Chem. Int. Ed. 2014, 54, 746–784. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, A.A.; Zamisa, S.J.; Omondi, B. Ag(I) complexes of imine derivatives of unexpected 2-thiophenemethyl homo-coupling and Bis-(E)-N-(furan-2-ylmethyl)-1-(quinolin-2-yl)methanimine. Molbank 2021, 2, 1235. [Google Scholar] [CrossRef]
- Castiñeiras, A.; Pedrido, R. Novel fluorescent cationic silver thiosemicarbazone clusters containing different eight-membered Ag4S4 metallacycles. Inorg. Chem. 2009, 48, 4847–4855. [Google Scholar] [CrossRef]
- Su, W.; Hong, M.; Weng, J.; Cao, R.; Lu, S. A Semiconducting Lamella Polymer [{Ag (C5H4NS)} n] with a Graphite-Like Array of Silver (I) Ions and Its Analogue with a Layered Structure. Angew. Chem. Int. Ed. 2000, 39, 2911–2914. [Google Scholar] [CrossRef]
- Zhong, J.C.; Misaki, Y.; Munakata, M.; Kuroda-Sowa, T.; Maekawa, M.; Suenaga, Y.; Konaka, H. Silver (I) Coordination Polymer of 2, 5-Bis-(4‘, 5‘-bis (methylthio)-1‘, 3‘-dithiol-2‘-ylidene)-1, 3, 4, 6-tetrathiapentalene (TTM-TTP) and Its Highly Conductive Iodine Derivative. Inorg. Chem. 2001, 40, 7096–7098. [Google Scholar] [CrossRef] [PubMed]
- Safin, D.A.; Mdluli, P.S.; Revaprasadu, N.; Ahmad, K.; Afzaal, M.; Helliwell, M.; O’Brien, P.; Shakirova, E.R.; Babashkina, M.G.; Klein, A. Nanoparticles and thin films of silver from complexes of derivatives of N-(diisopropylthiophosphoryl) thioureas. Chem. Mater. 2009, 21, 4233–4240. [Google Scholar] [CrossRef]
- Gao, S.; Chen, D.; Li, Q.; Ye, J.; Jiang, H.; Amatore, C.; Wang, X. Near-infrared fluorescence imaging of cancer cells and tumors through specific biosynthesis of silver nanoclusters. Sci. Rep. 2015, 4, 4384. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Tolufashe, G.F.; Mocktar, C.; Omondi, B. Ag(I) symmetrical N,N′-diarylformamidine dithiocarbamate PPh3 complexes: Synthesis, structural characterization, quantum chemical calculations and in vitro biological studies. Inorganica Chim. Acta 2021, 520, 120316. [Google Scholar] [CrossRef]
- Walker, M.; Parsons, D. The biological fate of silver ions following the use of silver-containing wound care products–a review. Int. Wound J. 2014, 11, 496–504. [Google Scholar]
- Mosconi, N.; Giulidori, C.; Velluti, F.; Hure, E.; Postigo, A.; Borthagaray, G.; Back, D.F.; Torre, M.H.; Rizzotto, M.J.C. Antibacterial, Antifungal, Phytotoxic, and Genotoxic Properties of Two Complexes of AgI with Sulfachloropyridazine (SCP): X-ray Diffraction of [Ag (SCP)] n. ChemMedChem 2014, 9, 1211–1220. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Haque, R.A.; Ahamed, M.B.K.; Majid, A.A.; Al-Rawi, S.S. Synthesis and anticancer activity of para-xylyl linked bis-benzimidazolium salts and respective Ag (I) N-heterocyclic carbene complexes. Med. Chem. Res. 2013, 22, 2455–2466. [Google Scholar] [CrossRef]
- Hemmert, C.; Fabié, A.; Fabre, A.; Benoit-Vical, F.; Gornitzka, H. Synthesis, structures, and antimalarial activities of some silver (I), gold (I) and gold (III) complexes involving N-heterocyclic carbene ligands. Eur. J. Med. Chem. 2013, 60, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Steel, P.J.; Fitchett, C.M. Metallosupramolecular silver (I) assemblies based on pyrazine and related ligands. Coord. Chem. Rev. 2008, 252, 990–1006. [Google Scholar] [CrossRef]
- Zhang, W.G.; Zhong, Y.; Tan, M.Y.; Liu, W.S.; Su, C.Y. A Novel Octahedral Hexasilver (I) Cluster: Ag6[SSCN (n-C4H9) 2]6. Chin. J. Chem. 2010, 20, 420–423. [Google Scholar] [CrossRef]
- Liu, N.; Fan, J.; Zhang, W.-G.; Yin, X.; Xie, M.-B. Bis (μ-N, N-diethyldithiocarbamato-κ3S, S′: S′) bis [(μ-N, N-diethyldithiocarbamato-κ2S, S′) silver (II)]. Acta Crystallogr. Sect. E 2006, 62, m2588–m2590. [Google Scholar] [CrossRef]
- Yin, X.; Xie, M.-B.; Zhang, W.-G.; Fan, J. Poly [(μ3-N, N-dibenzyldithiocarbamato-κ4S, S′: S: S′) silver (I)]. Acta Crystallogr. Sect. E 2007, 63, m2273. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Omondi, B.; Mocktar, C. Synthesis and structural studies of Ni(II) and Cu(II) N,N′-diarylformamidine dithiocarbamate complexes as antimicrobial and antioxidant agents. Polyhedron 2019, 170, 712–722. [Google Scholar] [CrossRef]
- Kishore, P.V.; Liao, J.-H.; Hou, H.-N.; Lin, Y.-R.; Liu, C.W. Ferrocene-functionalized Cu (I)/Ag (I) dithiocarbamate clusters. Inorg. Chem. 2016, 55, 3663–3673. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kido, A.; Uechi, T.; Yasukouchi, K. The Crystal and Molecular Structure of Silver (I) N, N-Diethyldithiocarbamate. Bull. Chem. Soc. Jpn. 1976, 49, 1271–1276. [Google Scholar] [CrossRef]
- Tong, M.C.; Chen, W.; Sun, J.; Ghosh, D.; Chen, S. Dithiocarbamate-capped silver nanoparticles. J. Phys. Chem. B 2006, 110, 19238–19242. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.T.; Bakar, S.A.; Saima, B.; Muhammad, B. Low temperature deposition of silver sulfide thin films by AACVD for gas sensor application. Appl. Surf. Sci. 2012, 258, 9610–9616. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Khaledi, H.; Tahir, A.A.; Ming, H.N.; Wijayantha, K.U.; Mazhar, M. Synthesis and characterization of silver diethyldithiocarbamate cluster for the deposition of acanthite (Ag2S) thin films for photoelectrochemical applications. Thin Solid Film. 2013, 536, 124–129. [Google Scholar] [CrossRef]
- Song, Y.-W.; Yu, Z.; Zhang, Q.-F. γ-Modification of poly [(N, N-diethyldithiocarbamato) silver (I)]. Acta Crystallogr. Sect. C 2006, 62, m214–m216. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Xie, M.-B.; Zhang, W.-G.; Fan, J.; Zeller, M. Hexakis (μ3-N, N-diisopropyldithiocarbamato) hexasilver (I)(6 Ag—Ag). Acta Crystallogr. Sect. E 2007, 63, m2063–m2064. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Olotu, F.A.; Soliman, M.; Mocktar, C.; Omondi, B. Formamidibe-based thiuram disulfides: Synthesis, structural characterization, biological studies and preliminary cheminformatics evaluation. J. Mol. Struct. 2020, 1219, 128553. [Google Scholar] [CrossRef]
- Bruker. APEXII; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Bruker. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Bruno, C.F.I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Streek, J.V.D.; Wood, P.A. Mercury 2.0-new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar]
- Mohammad, A.; Varshney, C.; Nami, S.A. Synthesis, characterization and antifungal activities of 3d-transition metal complexes of 1-acetylpiperazinyldithioc arbamate, M (acpdtc) 2. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2009, 73, 20–24. [Google Scholar] [CrossRef]
- Siddiqi, K.; Khan, S.; Nami, S.A.; El-Ajaily, M. Polynuclear transition metal complexes with thiocarbohydrazide and dithiocarbamates. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2007, 67, 995–1002. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Omondi, B. Mercury(II) N,N′-diarylformamidine dithiocarbamate as single-source precursors for the preparation of oleylamine-capped HgS nanoparticles. Transit. Met. Chem. 2020, 45, 391–402. [Google Scholar] [CrossRef]
- Gölcü, A. Transition metal complexes of propranolol dithiocarbamate: Synthesis, characterization, analytical properties and biological activity. Transit. Met. Chem. 2006, 31, 405–412. [Google Scholar] [CrossRef]
- Korneeva, E.; Ivanov, A.; Gerasimenko, A.; Loseva, O.; Novikova, E.; Ivanov, M. Hexanuclear Silver (I) Hexamethylene Dithiocarbamate Cluster [Ag 6{S 2CN(CH2)6} 6]·2CH 2 Cl 2: Preparation, Molecular Structure (Manifestation of Argentophilic Interaction), and Thermal Behavior. Russ. J. Gen. Chem. 2019, 89, 1642–1648. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Omondi, B.; Mocktar, C. Co (III) N, N′-diarylformamidine dithiocarbamate complexes: Synthesis, characterization, crystal structures and biological studies. Appl. Organomet. Chem. 2020, 34, e5610. [Google Scholar] [CrossRef]
- Lane, A.C.; Vollmer, M.V.; Laber, C.H.; Melgarejo, D.Y.; Chiarella, G.M.; Fackler, J.P.; Yang, X.; Baker, G.A.; Walensky, J.R. Multinuclear Copper(I) and Silver(I) Amidinate Complexes: Synthesis, Luminescence, and CS2 Insertion Reactivity. Inorg. Chem. 2014, 53, 11357–11366. [Google Scholar] [CrossRef]
- Huang, Z.; Lei, X.; Hong, M.; Liu, H. Synergism in a transition metal cluster compound. Crystal and molecular structure of a polysilver cluster molecule with an unusual bridging sulfur atom, Ag11S (Et2dtc) 9. Inorg. Chem. 1992, 31, 2990–2991. [Google Scholar] [CrossRef]
- Liu, C.; Liao, P.-K.; Fang, C.-S.; Saillard, J.-Y.; Kahlal, S.; Wang, J.-C. An eleven-vertex deltahedron with hexacapped trigonal bipyramidal geometry. Chem. Commun. 2011, 47, 5831–5833. [Google Scholar] [CrossRef] [Green Version]
- Prakasam, B.A.; Lahtinen, M.; Peuronen, A.; Muruganandham, M.; Kolehmainen, E.; Haapaniemi, E.; Sillanpää, M. Spectral and structural studies on Ni(II) dithiocarbamates: Nickel sulfide nanoparticles from a dithiocarbamate precursor. Inorg. Chim. Acta 2015, 425, 239–246. [Google Scholar] [CrossRef]


| 1 | 2 | |
|---|---|---|
| Empirical formula | C156H210Ag6N12S12 | C40H46Ag2N4S4 |
| Formula weight | 3285.29 | 926.79 |
| Crystal system | Trigonal | Triclinic |
| Space group | R-3: H | P-1 |
| a/Å | 30.0941(6) | 8.1650(5) |
| b/Å | 30.0941(6) | 15.5778(9) |
| c/Å | 50.6555(11) | 16.2365(10) |
| α/° | 90 | 77.7000(10) |
| β/° | 90 | 87.3590(2) |
| γ/° | 120 | 83.1580(3) |
| Space group no. | 148 | 2 |
| Volume/Å3 | 39,730(2) | 2002.9(2) |
| Z | 9 | 2 |
| ρcalcg/cm3 | 1.236 | 1.537 |
| μ/mm−1 | 0.841 | 1.220 |
| F(000) | 15,336 | 944 |
| Crystal size/mm3 | 0.320 × 0.210 × 0.140 | 0.32 × 0.19 × 0.11 |
| 2θ range for data collection/° | 1.614 × 26.389 | 1.655 to 26.999 |
| Index ranges | −31 ≤ h ≤ 37 | −9 ≤ h ≤ 10 |
| −37 ≤ h ≤ 24 | −19 ≤ k ≤ 18 | |
| −62 ≤ l ≤ 61 | −20 ≤ l ≤ 12 | |
| Reflections collected | 91,820 | 6320 |
| Independent reflections | 17,643 | 8656 |
| Data/restraints/parameters | 17,643/0/862 | 8657/7/1474 |
| Goodness-of-fit on F2 | 0.935 | 0.983 |
| Final R indexes [I > = 2σ(I)] | R1 = 0.0409 wR2 = 0.0868 | R1 = 0.0610 wR2 = 0.110 |
| Final R indexes [all data] | R1 = 0.0869, wR2 = 0.1009 | R1 = 0.0610, wR2 = 0.1196 |
| Largest diff peak & hole (e Å−3) | 1.597 and −0.799 | 1.551 and 0.879 |
| 1 | Polymorph | |
|---|---|---|
| Empirical formula | C156H210Ag6N12S12 | C174H262Ag6N12S12 |
| Formula weight | 3285.29 | 3553.88 |
| Crystal system | Trigonal | Cubic |
| Space group | R | Ia |
| a/Å | 30.0941(6) | 32.283(4) |
| b/Å | 30.0941(6) | 32.283(4) |
| c/Å | 50.6555(11) | 32.283(4) |
| α/° | 90 | 90 |
| β/° | 90 | 90 |
| γ/° | 120 | 90 |
| Volume/Å3 | 39,730(2) | 33,644.8(2) |
| Z | 9 | 8 |
| ρcalcg/cm3 | 1.236 | 1.403 |
| Largest diff. peak & hole (e Å−3) | 1.597 and −0.799 | 1.551 and 0.879 |
| Bond distances (Å) | 1 | Polymorph | 2 |
|---|---|---|---|
| Ag—Ag | 2.9545(4) | 3.0001(1) | 2.8829(6) |
| Ag—Ag | 2.9695(4) | 3.0001(1) | 3.1932(5) |
| Ag—Ag | 3.3452(4) | 3.0001(1) | 3.0644(5) |
| Ag—S | 2.4857(11) | 2.4777(1) | 2.5029(9) |
| Ag—S | 2.5322(9) | 2.5007 (1) | 2.4805(10) |
| C—S | 1.681(4) | 1.6962(1) | 1.682(5) |
| C—S | 1.739(4) | 1.7404(1) | 1.723(4) |
| C—N | 1.362(5) | 1.3632(1) | 1.366(4) |
| C—N | 1.371(4) | 1.4639(1) | 1.406(5) |
| Bond angles (°) | |||
| Ag—S—Ag | 85.03(3) | 82.20 | 74.38(3) |
| S—Ag—S | 107.07(4) | 109.29 | 111.97(4) |
| Ag—Ag—S | 77.81(2) | 80.27 | 88.90(2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladipo, S.D.; Omondi, B. N,N′-Diarylformamidine Dithiocarbamate Ag(I) Cluster and Coordination Polymer. Molbank 2022, 2022, M1327. https://doi.org/10.3390/M1327
Oladipo SD, Omondi B. N,N′-Diarylformamidine Dithiocarbamate Ag(I) Cluster and Coordination Polymer. Molbank. 2022; 2022(1):M1327. https://doi.org/10.3390/M1327
Chicago/Turabian StyleOladipo, Segun D., and Bernard Omondi. 2022. "N,N′-Diarylformamidine Dithiocarbamate Ag(I) Cluster and Coordination Polymer" Molbank 2022, no. 1: M1327. https://doi.org/10.3390/M1327
APA StyleOladipo, S. D., & Omondi, B. (2022). N,N′-Diarylformamidine Dithiocarbamate Ag(I) Cluster and Coordination Polymer. Molbank, 2022(1), M1327. https://doi.org/10.3390/M1327







