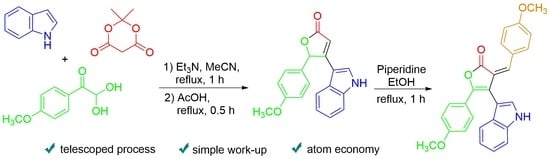
Multicomponent Approach to the Synthesis of 4-(1H-indol-3-yl)-5-(4-methoxyphenyl)furan-2(5H)-one
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
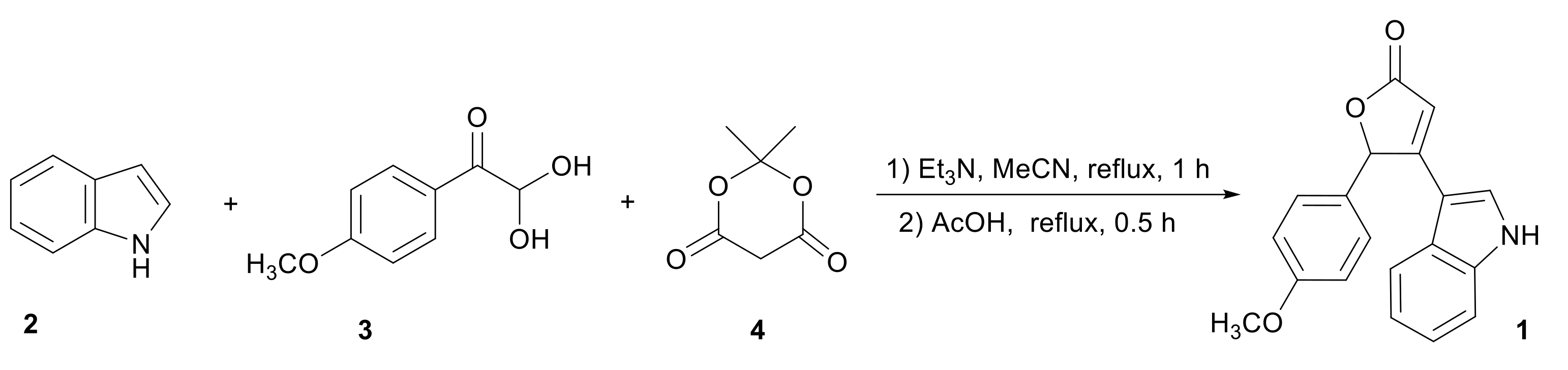
3.1. Synthesis of 4-(1H-Indol-3-yl)-5-(4-methoxyphenyl)furan-2(5H)-one 1
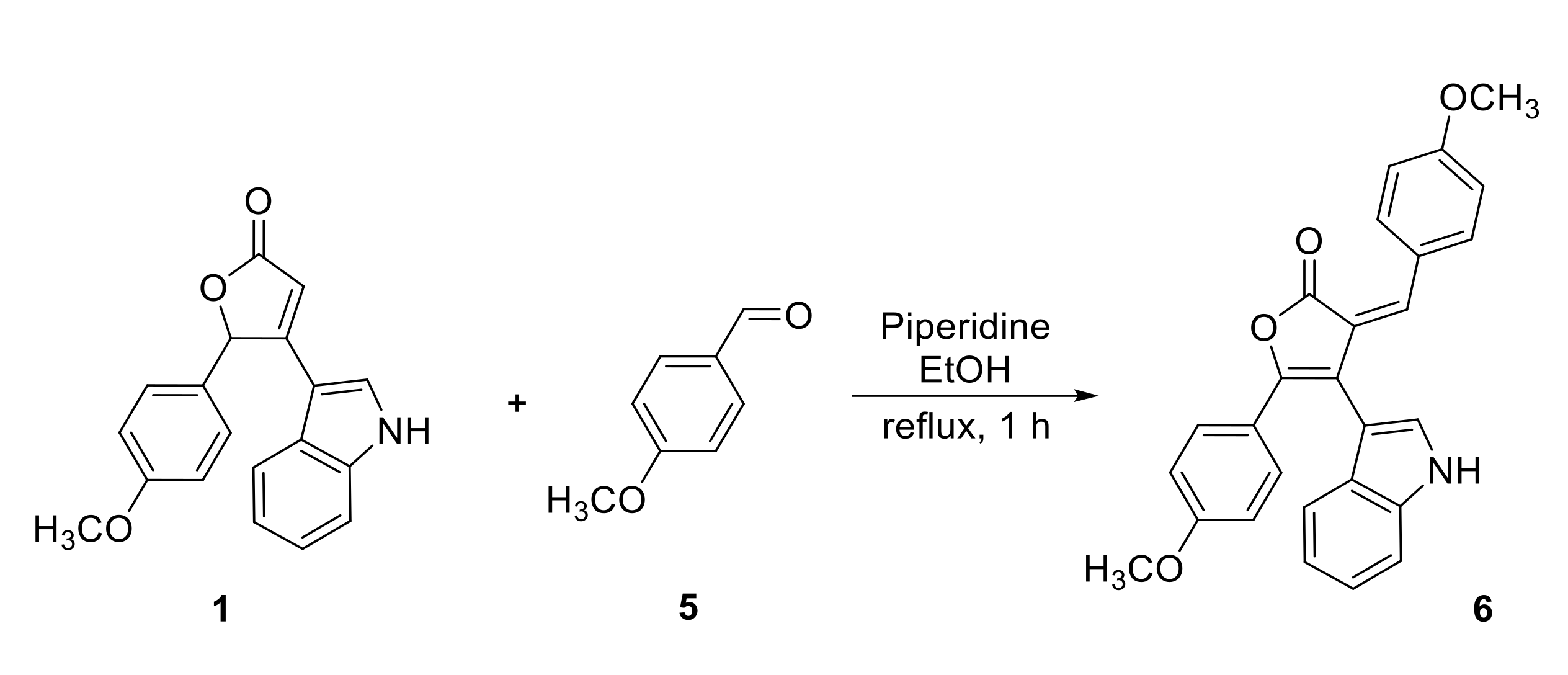
3.2. Synthesis of 4-(1H-Indol-3-yl)-3-(4-methoxybenzylidene)-5-(4-methoxyphenyl)furan-2(3H)-one 6
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avetisyan, A.A.; Dangyan, M.T. The Chemistry of γ-Butenolides. Russ. Chem. Rev. 1977, 46, 643–656. [Google Scholar] [CrossRef]
- Mao, B.; Fañanás-Mastral, M.; Feringa, B.L. Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones. Chem. Rev. 2017, 117, 10502–10566. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Thirupathi, B. Strategies for the Construction of γ-Spirocyclic Butenolides in Natural Product Synthesis. Org. Biomol. Chem. 2020, 18, 5287–5314. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Khalifa, S.A.M.; Taher, E.A.; Farag, M.A.; Saeed, A.; Gamal, M.; Hegazy, M.-E.F.; Youssef, D.; Musharraf, S.G.; Alajlani, M.M.; et al. Cardenolides: Insights from Chemical Structure and Pharmacological Utility. Pharmacol. Res. 2019, 141, 123–175. [Google Scholar] [CrossRef]
- Michalak, M.; Michalak, K.; Wicha, J. The Synthesis of Cardenolide and Bufadienolide Aglycones, and Related Steroids Bear-ing a Heterocyclic Subunit. Nat. Prod. Rep. 2017, 34, 361–410. [Google Scholar] [CrossRef]
- Kim, Y.; Nam, N.-H.; You, Y.-J.; Ahn, B.-Z. Synthesis and Cytotoxicity of 3,4-Diaryl-2(5H)-Furanones. Bioorg. Med. Chem. Lett. 2002, 12, 719–722. [Google Scholar] [CrossRef]
- Husain, A.; Alam, M.M.; Shaharyar, M.; Lal, S. Antimicrobial Activities of Some Synthetic Butenolides and Their Pyrrolone Derivatives. J. Enzyme Inhib. Med. Chem. 2010, 25, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Ali, Y.; Alam, M.S.; Hamid, H.; Husain, A.; Shafi, S.; Dhulap, A.; Hussain, F.; Bano, S.; Kharbanda, C.; Nazreen, S.; et al. Design and Synthesis of Butenolide-Based Novel Benzyl Pyrrolones: Their TNF-α Based Molecular Docking with In Vivo and in Vitro. Anti-Inflammatory Activity. Chem. Biol. Drug Des. 2015, 86, 619–625. [Google Scholar] [CrossRef]
- Yang, C.-P.; Huang, G.-J.; Huang, H.-C.; Chen, Y.-C.; Chang, C.-I.; Wang, S.-Y.; Chen, I.-S.; Tseng, Y.-H.; Chien, S.-C.; Kuo, Y.-H. A New Butanolide Compound from the Aerial Part of Lindera Akoensis with Anti-Inflammatory Activity. Molecules 2012, 17, 6585–6592. [Google Scholar] [CrossRef]
- Igarashi, Y.; Ikeda, M.; Miyanaga, S.; Kasai, H.; Shizuri, Y.; Matsuura, N. Two Butenolides with PPARα Agonistic Activity from a Marine-Derived Streptomyces. J. Antibiot. 2015, 68, 345–347. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Jia, W.; Xi, Q.; Chen, Y.; Wang, X.; Yin, D. Diversity-Oriented Synthesis of Heterocycles: Al(OTf)3-Promoted Cascade Cyclization and Ionic Hydrogenation. J. Org. Chem. 2018, 83, 1387–1393. [Google Scholar] [CrossRef]
- Shimizu, K.; Kanno, S.; Kon, K. Hydrogenation of Levulinic Acid to γ-Valerolactone by Ni and MoOx Co-Loaded Carbon Catalysts. Green Chem. 2014, 16, 3899–3903. [Google Scholar] [CrossRef]
- Liu, Y.; Song, F.; Guo, S. Cleavage of a Carbon−Carbon Triple Bond via Gold-Catalyzed Cascade Cyclization/Oxidative Cleavage Reactions of (Z)-Enynols with Molecular Oxygen. J. Am. Chem. Soc. 2006, 128, 11332–11333. [Google Scholar] [CrossRef]
- Hyde, A.M.; Buchwald, S.L. Synthesis of 5,5-Disubstituted Butenolides Based on a Pd-Catalyzed γ-Arylation Strategy. Org. Lett. 2009, 11, 2663–2666. [Google Scholar] [CrossRef] [Green Version]
- Stache, E.E.; Ertel, A.B.; Rovis, T.; Doyle, A.G. Generation of Phosphoranyl Radicals via Photoredox Catalysis Enables Voltage–Independent Activation of Strong C–O Bonds. ACS Catal. 2018, 8, 11134–11139. [Google Scholar] [CrossRef]
- Reddy, M.S.; Thirupathi, N.; Babu, M.H.; Puri, S. Synthesis of Substituted 3-Iodocoumarins and 3-Iodobutenolides via Electrophilic Iodocyclization of Ethoxyalkyne Diols. J. Org. Chem. 2013, 78, 5878–5888. [Google Scholar] [CrossRef]
- Fu, C.; Ma, S. Efficient Preparation of 4-Iodofuran-2(5H)-Ones by Iodolactonisation of 2,3-Allenoates with I2. Eur. J. Org. Chem. 2005, 18, 3942–3945. [Google Scholar] [CrossRef]
- Kawamata, Y.; Hashimoto, T.; Maruoka, K. A Chiral Electrophilic Selenium Catalyst for Highly Enantioselective Oxidative Cyclization. J. Am. Chem. Soc. 2016, 138, 5206–5209. [Google Scholar] [CrossRef]
- Suero, M.G.; De la Campa, R.; Torre-Fernández, L.; García-Granda, S.; Flórez, J. Enantioselective Multicomponent Synthesis of Fused 6-5 Bicyclic 2-Butenolides by a Cascade Heterobicyclisation Process. Chem. Eur. J. 2012, 18, 7287–7295. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, M.; Hu, X.; Li, B.-G.; Ji, J.-X. Gold-Catalyzed Three-Component Tandem Process: An Efficient and Facile Assembly of Complex Butenolides from Alkynes, Amines, and Glyoxylic Acid. J. Am. Chem. Soc. 2010, 132, 7256–7257. [Google Scholar] [CrossRef]
- Abbiati, G.; Rossi, E. Silver and Gold-Catalyzed Multicomponent Reactions. Beilstein J. Org. Chem. 2014, 10, 481–513. [Google Scholar] [CrossRef] [Green Version]
- Nechaev, A.; Peshkov, A.; Peshkov, V.; Van der Eycken, E. The Application of Multicomponent Ugi and Passerini Reactions for the One-Pot Synthesis of Pyrrolones and Butenolides. Synthesis 2016, 48, 2280–2286. [Google Scholar] [CrossRef]
- Beck, B.; Magnin-Lachaux, M.; Herdtweck, E.; Dömling, A. A Novel Three-Component Butenolide Synthesis. Org. Lett. 2001, 3, 2875–2878. [Google Scholar] [CrossRef]
- Khan, G.A.; War, J.A.; Kumar, A.; Sheikh, I.A.; Saxena, A.; Das, R. A Facile Synthesis of Novel Indole Derivatives as Poten-tial Antitubercular Agents. J. Taibah Univ. Sci. 2017, 11, 910–921. [Google Scholar] [CrossRef] [Green Version]
- Gobinath, P.; Packialakshmi, P.; Vijayakumar, K.; Abdellattif, M.H.; Shahbaaz, M.; Idhayadhulla, A.; Surendrakumar, R. Synthesis and Cytotoxic Activity of Novel Indole Derivatives and Their In Silico Screening on Spike Glycoprotein of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 255. [Google Scholar] [CrossRef]
- Sachdeva, H.; Mathur, J.; Guleria, A. Indole Derivatives as Potential Anticancer Agents: A Review. J. Chil. Chem. Soc. 2020, 65, 4900–4907. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, P.; Pathak, D. Biological Importance of the Indole Nucleus in Recent Years: A Comprehensive Review. J. Heterocycl. Chem. 2010, 47, 491–502. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Medicinal Chemistry of Indole Derivatives: Current to Future Therapeutic Prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef]
- Lichitsky, B.V.; Melekhina, V.G.; Komogortsev, A.N.; Minyaev, M.E. A New Multicomponent Approach to the Synthesis of Substituted Furan-2(5H)-Ones Containing 4H-Chromen-4-One Fragment. Tetrahedron Lett. 2020, 61, 152602. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komogortsev, A.N.; Lichitsky, B.V.; Melekhina, V.G. Multicomponent Approach to the Synthesis of 4-(1H-indol-3-yl)-5-(4-methoxyphenyl)furan-2(5H)-one. Molbank 2021, 2021, M1292. https://doi.org/10.3390/M1292
Komogortsev AN, Lichitsky BV, Melekhina VG. Multicomponent Approach to the Synthesis of 4-(1H-indol-3-yl)-5-(4-methoxyphenyl)furan-2(5H)-one. Molbank. 2021; 2021(4):M1292. https://doi.org/10.3390/M1292
Chicago/Turabian StyleKomogortsev, Andrey N., Boris V. Lichitsky, and Valeriya G. Melekhina. 2021. "Multicomponent Approach to the Synthesis of 4-(1H-indol-3-yl)-5-(4-methoxyphenyl)furan-2(5H)-one" Molbank 2021, no. 4: M1292. https://doi.org/10.3390/M1292
APA StyleKomogortsev, A. N., Lichitsky, B. V., & Melekhina, V. G. (2021). Multicomponent Approach to the Synthesis of 4-(1H-indol-3-yl)-5-(4-methoxyphenyl)furan-2(5H)-one. Molbank, 2021(4), M1292. https://doi.org/10.3390/M1292