Abstract
Star-shaped compounds are widely recognized as emerging materials for optical and electrical applications and as scaffolds of discotic liquid crystal. While the C3-symmetrical tri(phenylthienyl)benzene is the core for several electroopotical materials, no liquid crystal with this scaffold has yet been reported. Acid-catalyzed cyclocondensation of bromoacetylthiophene gives a C3-symmetrical star, threefold Suzuki coupling results in extension of the conjugated system. With 3,4-didodecylocyphenyl boronic acid, a star with a large rigid conjugated system and flexible aliphatic periphery is obtained. Differentials scanning calorimetry and polarized optical microscopy reveal an enantiotropic mesophase from 66 °C to 106 °C.
1. Introduction
Thiophene is a very prominent building block for the synthesis of organic electronic materials [1,2] and a large variety of oligo- and polythiophenes have been prepared and successfully applied as active material in electronic devices, e.g., OLEDs, OFETs, OVPs and sensors [3,4,5]. The alignment of the materials within the conductive layer is a highly competitive challenge in plastic electronics. Jiang et al. [6] presented the targeted electrochemical polymerization to optimally align the π-systems on the electrode. Discotic liquid crystals (DLCs) offer another strategy for orientation and targeted alignment, self-assembly of their large aromatic cores can form conductive columns [7,8,9]. Whereas a large number of star-shaped [10,11] and discotic [12,13] π-conjugated systems have been investigated, compounds with a thiophene based core appear only scarcely [13,14]. 1,3,5-Tris-(2-thienyl)benzene has been prepared via cyclocondensation of 2-acetylthiophenes [15], a recent approach is based on the addition of benzenetri(methylthiol) to diynes [16]. Extension of the conjugated system is possible via pd-catalyzed coupling reactions [17]. A few optoelectronic materials based on 1,3,5-trithienylbenzene have been extensively investigated [18,19,20,21,22]. Though this scaffold has even been used for the construction of catalysts [23] and gold nanoparticles [24], mesomorphous materials based on 1,3,5-trithienylbenzene are unknown. Here we report the synthesis of tris(didodecyloxyphenylthienyl)benzene, a π-conjugated star and the first discotic liquid crystal with this core.
2. Results
2.1. Synthesis
Retrosynthetic analysis of the title compound gives different possibilities, either the combination of the arms with formation of the central ring or the stepwise approach, construction of the arms in successive threefold coupling reactions. The first is an alkyne cyclotrimerization of ethynyl-substituted alkoxyphenyl thiophene. This generates the central ring, but with a notoriously poor regioselectivity. Starting with a 1,3,5-funtionalized benzene allows the second approach. This is a sequence of three three-fold reactions: a three-fold Suzuki-Miyaura coupling, three-fold bromination followed by a second threefold coupling reaction [25]. 1,3,5-Triarylbenzenes are easily prepared via cyclocondensation of acetylarenes [26]. Though this method is applicable to acetylthiophene [27], the cyclization of the analogous 5-bromo-2-acetylthiophene failed [28]. Nevertheless, Tokárová reported a 28% yield of the trimer when SiCl4/ethanol was used as dehydrating agent [29]. In an extensive study, we could raise the yield up to 37%. The connection of the dialkoxyphenyl rings to the thiophenes was performed via Suzuki-Miyaura coupling of 3,4-di(dodecyloxy)phenylboronic acid [30] to the tribromo trithienyl benzene (Scheme 1).
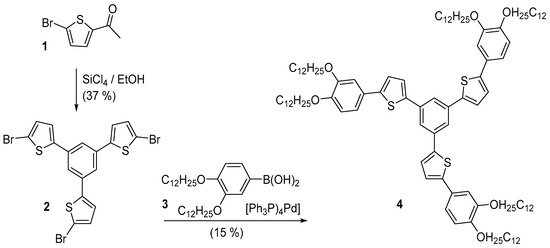
Scheme 1.
Synthesis of 2-{3,5-Bis-[5-(3,4-didodecyloxyphenyl)thien-2-yl]phenyl}-5-(3,4-di-dodecyloxyphenyl)thiophene via Suzuki coupling on the cyclotrimer of 2-acetyl-5-bromothiophene.
2.2. Experimental Procedures: Synthesis of 1,3,5-Tris(5-bromothienyl)benzene 2
2-Acetyl-5-bromothiophene 1 (500 mg) was dissolved in dry ethanol (5 mL) at 0 °C in a N2 atmosphere. SiCl4 (0.7 mL, 2.5 eq) was added dropwise over 10 min to the stirred solution and stirring was continued for 24 h at ambient temperature. Two further portions SiCl4 (each: 0.42 mL, 1.5 eq) were added after 24 and 48 h. the mixture was diluted with ice/water (5 mL) and extracted with toluene (3 × 5 mL). Washing the combined organic solutions with brine, drying with MgSO4, evaporation of solvent and chromatography (SiO2, petroleum ether/toluene = 5/1 over a short column yielded, after recrystallization from ethanol, the tribomide 2 (137 mg, 37 %) as yellow solid with m.p. = 176–178 °C. 1H NMR (300 MHz, CDCl3, 298 K): δ = 7.52 (m, 3 H); 7.14 (m, 3 H), 7,97 (d, 3 H, J = 3.9 Hz). Whereas 1H-NMR data are identical to the data reported from different groups, e.g., in ref. [28,31], the melting point of 2 is about 64° higher, as shown in the published data. As Rebourt et al. [32] reported a melting point of 183 °C, we assume polymorphism of 2.
Experimental Procedure: Synthesis of 2.3.2-{3,5-Bis-[5-(3,4-didodecyloxyphenyl)thien-2- yl]phenyl}-5-(3,4-didodecyloxyphenyl)thiophene 4
A Schlenk tube was charged with tris- 1,3,5-(5′ bromothien 2 yl)benzene 2 (72 mg, 128 µmol, 1.0 eq), 3,4 didodecyloxypenylbronic acid 3 (208 mg, 424 µmol, 1.0 eq), and K2CO3 (80 mg, 577 µmol, 4.5 eq). After a mixture of toluene and water (2.5/1.25 mL) had been added, the mixture was degassed with nitrogen at 50 °C and tetrakistriphenylphosphin palladium(0) (8.0 mg, 7 µmol, 5.4 mol-%) was added and the mixture refluxed for 48 h. After dilution of the mixture with water (20 mL) and toluene (20 mL), the organic layer was separated and the aqueous layer was extracted with toluene (3 × 5 mL). The pooled organic solution was washed with hydrochloric acid (2 M, 10 mL) and water (15 mL), dried over MgSO4 and the solvent was evaporated. The remaining brownish wax was purified by column chormatography on silica with toluene/petroleum ether as eluent. A gradient from 0:1 to 1:0 was applied. The main fraction was recrystallized from propanol-2 and a second chromatography on alumina with cyclohexane/toluene gradient and, finally, recrystallization from propanol-2 gave 31 mg (15%) of the title compound 4 with m.p. = 68 °C. Rf = 0.74 (SiO2, CH2Cl2/petroleum ether 1:1). 1H NMR (300 MHz, CDCl3, 298 K, HSQC, HMBC): δ = 7.74 (s, 3H, H1), 7.39 (d, 3J = 3.67 Hz, 3H, H4), 7.17–7.22 (m, 9H, H5,8,12), 6.96 (d, 3J = 9.29 Hz, 3H, H11), 4.01–4.11 (m, 12H, H13,25), 1.79–1.91 (m, 12H, H14,26), 1.27–1.54 (m, 118H, H15-23,27–35), 0.84–0.90 (m, 18H, H24-36). (Figure S2). 13C NMR (75 MHz, CDCl3, 298 K, HSQC, HMBC): δ = 149.52 (C10), 149.33 (C9), 144.67 (C6), 141.80 (C2), 135.83 (C3), 127.48 (C7), 124.77 (C4), 123.22 (C5), 121.70 (C1), 118.70 (C12), 114.15 (C11), 111.86 (C8), 69.61 (C13/21), 69.52 (C13/21), 32.09, 29.87, 29.81, 29.62, 29.61, 29.54, 29.51, 29.45, 26.24, 26.20, 22.86 (C14-23,26–35), 14.29(C24,36). (Figure S3). IR (ATR)/[cm−1]: 2919, 2849, 1586, 1516, 1464, 1389, 1322, 1251, 1139, 1019, 794, 721, 671. HR-MS (APCI): calcd.: 1657.2003; found: 1657.2313 [M]+; 1658,2371 [M + H]+. Mass spectrometry reveald traces of an impurity with m/z = 1213,8109. This corresponds to a product resulting from twofold Suzuki couplind and a hydro-debromination on the third branch, calculated m/z = 1213.8313.
2.3. Mesomorphism
The combination of a rigid π-conjugated core composed of seven aromatic rings and a flexible aliphatic periphery with six dodecyl chains results in an anisotropy of the molecular structure of 4. Polarized optical microscopy shows birefringence of the mobile mesophase (Figure 1). Differential scanning calorimetry reveals an enantiotropic mesomorphism. The second heating scan (10 K/min) shows a melting peak at 68.0 °C (onset: 65.5 °C) and a clearing transition with onset at 99.9 °C and maximum at 106.6 °C (Figure S7). Both transitions appear at temperatures about ΔT = 10 °C higher than their reverse counterparts in the cooling scan. A peculiarity should be mentioned: While melting enthalpies are generally higher than the associated clearing transition enthalpies, the values found for 4 are ΔH = 15.7 kJ/mol for melting and ΔH = 24.0 kJ/mol for clearing. A similar behavior has been observed for structural congeners with a tris-1,3,4-oxadiazolyltriazine center [33].
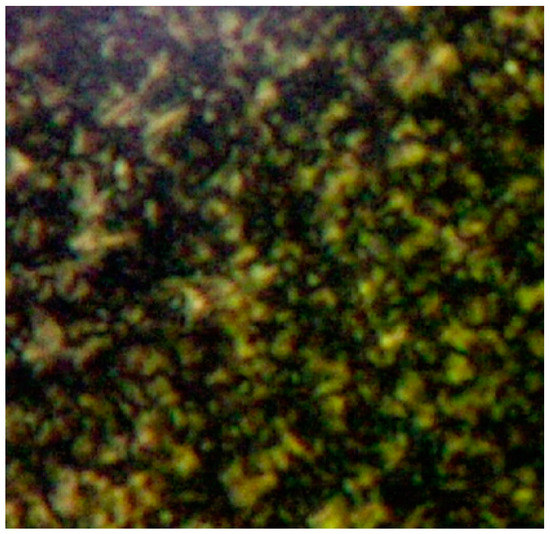
Figure 1.
Picture of birefringent mesophase with mosaic-like texture, observed via polarized optical microscopy of 4 in a heating cycle at 103 °C.
3. Materials and Methods
General Information: Commercially available reagents were used without further purification unless otherwise indicated; solvents and gases were dried by standard procedures. 1H- and 13C-NMR spectra: Bruker AC 300 (300 MHz) (Karlsruhe, Germany), Bruker AV 400 (400 MHz) (Karlsruhe, Germany), and Bruker ARX 400 (400 MHz) (Karlsruhe, Germany), solvents CDCl3. Chemical shifts are expressed as δ values in ppm, coupling constants are given in Hz. Assignments of 1H and 13C signals on the basis of HSQC, and HMBC experiments. Melting points: Stuart Scientific SMP3. DSC: Perkin Elmer (Rodgau, Germany), DSC 7, IR: JASCO 4100 FT-IR (ATR) (Pfungstadt, Germany), HR-ESI: Thermo QExactive Orbitrap MS NaICsI as reference. Polarized microscopy: Olympus BX51, Color View Olympus camera (Shinjuku, Japan), heating table Linkam LTS 350 for temperature regulation.
4. Conclusions
A combination of cyclocondensation and Suzuki-Miyaura coupling gives a short access to the first discotic liquid crystal with a 1,3,5-tris(phenylthienyl)benzene core.
Supplementary Materials
The following are available online, Figure S1: IR-spectrum, Figure S2: 1H-NMR-spectrum, Figure S3: 13C-NMR spectrum, Figure S4: 1H-1H-COSY NMR spectrum, Figure S5: HMBC NMR spectrum, Figure S6: HSQC NMR spectrum, Figure S7: DSC, Figure S8: POM.
Author Contributions
Conceptualization, M.J. and H.D.; methodology, M.J.; resources, H.D.; data curation, M.J.; writing—original draft preparation, H.D.; writing—review and editing, project administration, H.D.; funding acquisition, H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deutsche Forschungsgemeinschaft, grant number De 515/12-1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Müllen, K.; Wegner, G. Electronic Materials: The Oligomer Approach; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Geoghegan, M.; Hadziioannou, G. Polymer Electronics; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Bauerle, P.; Mitschke, U.; Gruner, G.; Rimmel, G. Structure-property relationships in functional conjugated oligomers. Pure Appl. Chem. 1999, 71, 2153–2160. [Google Scholar] [CrossRef]
- Maity, S.; Datta, S.; Mishra, M.; Banerjee, S.; Das, S.; Chatterjee, K. Poly(3,4 ethylenedioxythiophene)-tosylate-Its synthesis, properties and various applications. Polym. Adv. Technol. 2021, 32, 1409–1427. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Narayan, T.; Solanki, S.; Malhotra, B.D. Recent advances of conducting polymers and their composites for electrochemical biosensing applications. J. Funct. Biomater. 2020, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Huang, N.; Chen, Y.; Qin, L.; Xu, H.; Zhang, S.; Li, F.; Ma, Y.; Jiang, D. π-Conjugated Microporous Polymer Films: Designed Synthesis, Conducting Properties, and Photoenergy Conversions. Angew. Chem. 2015, 54, 13594–13598. [Google Scholar] [CrossRef] [PubMed]
- Wohrle, T.; Wurzbach, I.; Kirres, J.; Kostidou, A.; Kapernaum, N.; Litterscheidt, J.; Haenle, J.C.; Staffeld, P.; Baro, A.; Giesselmann, F.; et al. Discotic Liquid Crystals. Chem. Rev. 2016, 116, 1139–1241. [Google Scholar] [CrossRef] [PubMed]
- Grelet, E.; Bock, H. Control of the orientation of thin open supported columnar liquid crystal films by the kinetics of growth. Europhys. Lett. 2006, 73, 712–718. [Google Scholar] [CrossRef]
- Ikeda, T.; Adachi, H.; Fueno, H.; Tanaka, K.; Haino, T. Induced-Dipole-Directed, Cooperative Self-Assembly of a Benzotrithiophene. J. Org. Chem. 2017, 82, 10062–10069. [Google Scholar] [CrossRef]
- Detert, H.; Lehmann, M.; Meier, H. Star-shaped conjugated systems. Materials 2010, 3, 3218–3330. [Google Scholar] [CrossRef]
- Glang, S.; Rieth, T.; Borchmann, D.; Fortunati, I.; Signorini, R.; Detert, H. Arylethynyl-Substituted Tristriazolotriazines: Synthesis, Optical Properties, and Thermotropic Behavior. Eur. J. Org. Chem. 2014, 2014, 3116–3126. [Google Scholar] [CrossRef]
- Laschat, S.; Baro, A.; Steinke, N.; Giesselmann, F.; Hägele, C.; Scalia, G.; Judele, R.; Kapatsina, E.; Sauer, S.; Schreivogel, A.; et al. Discotic Liquid Crystals: From Tailor-Made Synthesis to Plastic Electronics. Angew. Chem. Int. Ed. 2007, 46, 4832–4887. [Google Scholar] [CrossRef]
- Demenev, A.; Eichhorn, S.H.; Taerum, T.; Perepichka, D.F.; Patwardhan, S.; Grozema, F.C.; Siebbeles, L.D.A.; Klenkler, R. Quasi Temperature Independent Electron Mobility in Hexagonal Columnar Mesophases of an H-Bonded Benzotristhiophene Derivative. Chem. Mater. 2010, 22, 1420–1428. [Google Scholar] [CrossRef]
- Tober, N.; Lehmann, M.; Detert, H. Synthesis, Thermal and Optical Properties of Tris(5-aryl-1,3,4-oxadiazolyl)benzo[1,2-b; 3,4-b’; 5,6-b’’]trithiophenes—New Discotic Liquid Crystals with Enormous Mesophase Ranges. Eur. J. Org. Chem. 2021, 25, 798–809. [Google Scholar] [CrossRef]
- Kotha, S.; Chakraborty, K.; Brahmachary, E. A general and simple method for the synthesis of star-shaped thiophene derivatives. Synlett 1999, 10, 1621–1623. [Google Scholar] [CrossRef]
- Klukas, F.; Perkampus, J.; Urselmann, D.; Mueller, T.J.J. Pseudo Five-Component Synthesis of 3-(Hetero)arylmethyl-2,5-di(hetero)-aryl-Substituted Thiophenes via Sonogashira-Glaser Cyclization Sequence. Synthesis 2014, 46, 3415–3422. [Google Scholar] [CrossRef]
- Kotha, S.; Kashinath, D.; Lahiri, K.; Sunoj, R.B. Synthesis of C3-Symmetric Nano-Sized Polyaromatic Compounds by Trimerization and Suzuki−Miyaura Cross-Coupling Reaction. Eur. J. Org. Chem. 2004, 19, 4003–4013. [Google Scholar] [CrossRef]
- Thomas, K.R.J.; Huang, T.-H.; Lin, J.T.; Pu, S.-C.; Cheng, Y.-M.; Hsieh, C.-C.; Tai, C.P. Donor–Acceptor Interactions in Red-Emitting Thienylbenzene-Branched Dendrimers with Benzothiadiazole Core. Chem. Europ. J. 2008, 14, 11231–11241. [Google Scholar] [CrossRef]
- Thomas, K.R.J.; Lin, J.T.; Tao, Y.-T.; Ko, C.-W. New Star-Shaped Luminescent Triarylamines: Synthesis, Thermal, Photophysical, and Electroluminescent Characteristics. Chem. Mater. 2002, 14, 1354–1361. [Google Scholar] [CrossRef]
- Lumpi, D.; Holzer, B.; Bintinger, J.; Horkel, E.; Waid, S.; Wanzenboeck, H.D.; Marchetti-Deschmann, M.; Hametner, C.; Bertagnolli, E.; Kymissis, I.; et al. Substituted triphenylamines as building blocks for star shaped organic electronic materials. New J. Chem. 2015, 39, 1840–1851. [Google Scholar] [CrossRef]
- Lin, Z.; Bjorgaard, J.; Yavuz, A.G.; Kose, M.E. Low Band Gap Star-Shaped Molecules Based on Benzothia(oxa)diazole for Organic Photovoltaics. J. Phys. Chem. C 2011, 115, 15097–15108. [Google Scholar] [CrossRef]
- Do, K.; Choi, H.; Lim, K.; Jo, H.; Cho, J.W.; Nazeeruddin, M.K.; Ko, J. Star-shaped hole transporting materials with a triazine unit for efficient perovskite solar cells. Chem. Commun. 2014, 50, 10971–10974. [Google Scholar] [CrossRef]
- Doba, T.; Matsubara, T.; Ilies, L.; Shang, R.; Nakamura, E. Homocoupling-free iron-catalysed twofold C–H activation/cross-couplings of aromatics via transient connection of reactants. Nat. Catal. 2019, 2, 400–406. [Google Scholar] [CrossRef]
- Quintana, C.; Morshedi, M.; Wang, H.; Du, J.; Cifuentes, M.P.; Humphrey, M.G. Exceptional Two-Photon Absorption in Alkynylruthenium–Gold Nanoparticle Hybrids. Nano Lett. 2019, 19, 756–760. [Google Scholar] [CrossRef]
- Mitchell, W.J.; Kopidakis, N.; Rumbles, G.; Ginley, D.S.; Shaheen, S.E. The synthesis and properties of solution processable phenyl cored thiophene dendrimers. J. Mater. Chem. 2005, 15, 4518–4528. [Google Scholar] [CrossRef]
- Engler, C.; Heine, H. Ueber die Einwirkung des Ammoniaks und seiner Abkömmlinge auf die Ketone bei Gegenwart von wasserentziehenden Substanzen. Berichte der Deutschen Chemischen Gesellschaft 1873, 6, 638–643. [Google Scholar] [CrossRef]
- Bui-Hoi, N.P.; Jacquignon, P.; Périn, F.; Delcey, M.C. Analogues hétérocycliques des triaryl-1,3,5-benzènes. C. R. Acad. Sci. Ser. C 1966, 262, 1237–1239. [Google Scholar]
- Kotha, S.; Todeti, S.; Gopal, M.B.; Datta, A. Synthesis and Photophysical Properties of C3-Symmetric Star-Shaped Molecules Containing Heterocycles Such as Furan, Thiophene, and Oxazole. ACS Omega 2017, 2, 6291–6297. [Google Scholar] [CrossRef] [PubMed]
- Andicsová-Eckstein, A.; Végh, D.; Krutošíková, A.; Tokárová, Z. Insights into the triple self-condensation reaction of thiophene-based methyl ketones and related compounds. Org. Chem. 2018, 124–139. [Google Scholar] [CrossRef]
- Yatabe, T.; Harbison, M.A.; Brand, J.D.; Wagner, M.; Muellen, K.; Samori, P.; Rabe, J.P. Extended triphenylenes: Synthesis, mesomorphic properties and molecularly resolved scanning tunneling microscopy images of hexakis(dialkoxyphenyl)triphenylenes and dodeca(alkoxy)tris(triphenylene)s. J. Mater. Chem. 2000, 10, 1519–1525. [Google Scholar] [CrossRef]
- Cherioux, F.; Guyard, L. Synthesis and Electrochemical Properties of Novel 1,3,5-Tris(oligothienyl)benzenes: A New Generation of 3D Recticulating Agents. Adv. Funct. Mater. 2001, 11, 305–309. [Google Scholar] [CrossRef]
- Rebourt, E.; Pépin-Donat, B.; Dinh, E. Routes towards three-dimensional fully conjugated conducting polymers: 1. Preparation of the kit of monomers. Polymer 1995, 36, 399–412. [Google Scholar] [CrossRef]
- Tober, N.; Rieth, T.; Lehmann, M.; Detert, H. Synthesis, Thermal, and Optical Properties of Tris(5-aryl-1,3,4-oxadiazol-2-yl)- 1,3,5-triazines, New Star-shaped Fluorescent Discotic Liquid Crystals. Chemistry 2019, 25, 15295–15304. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).