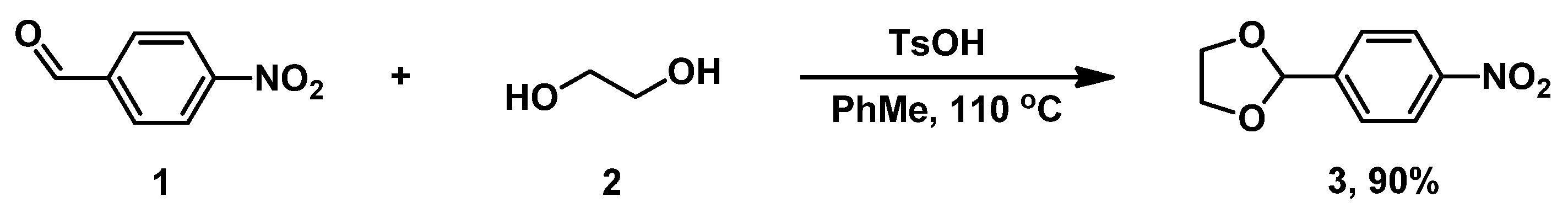Abstract
A simple approach to synthesizing 1,2-bis(4-(1,3-dioxolan-2-yl)phenyl)diazene oxide was developed in this study, based on glucose as an eco-friendly reductant.
1. Introduction
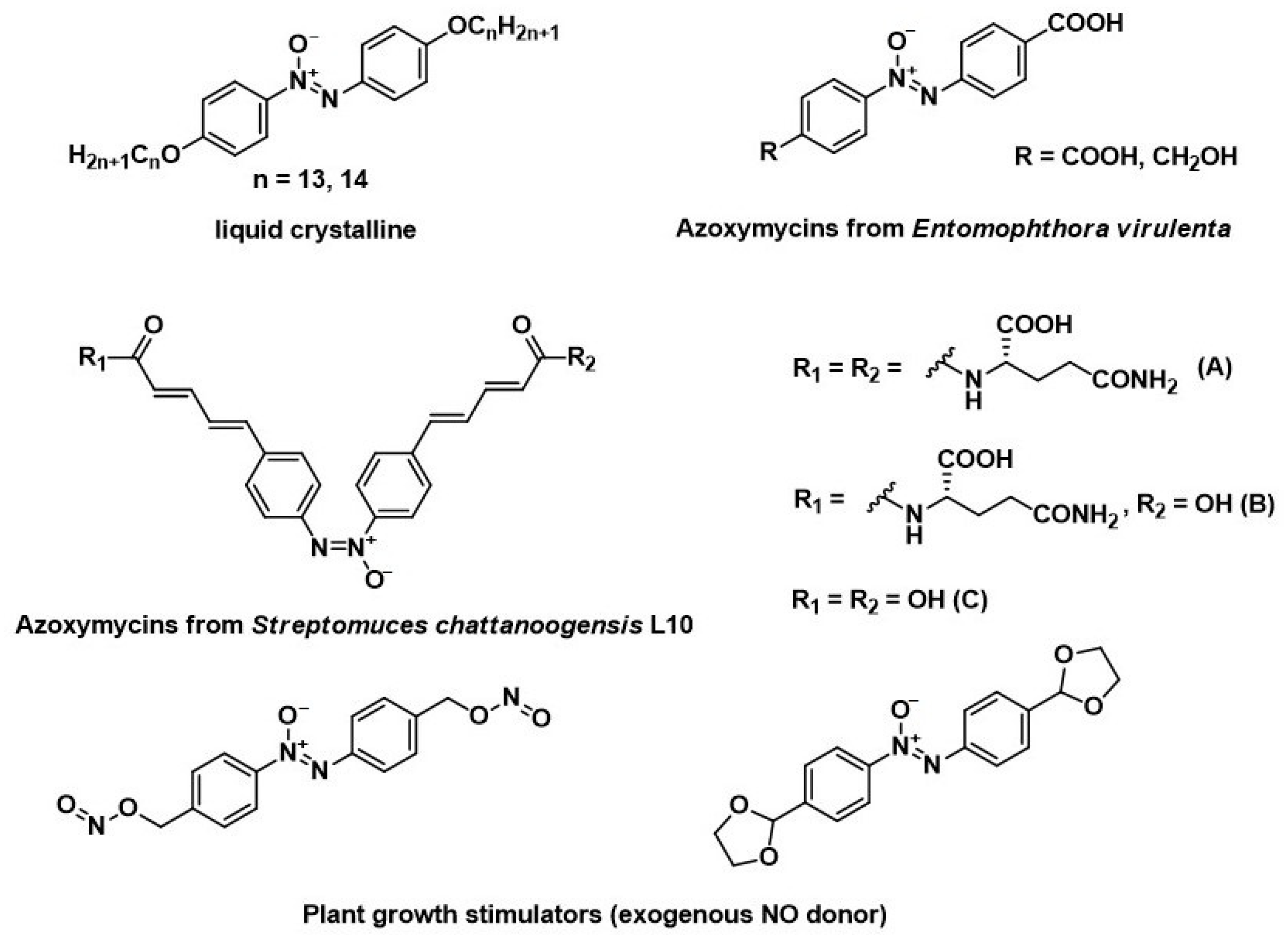
Azoxybenzenes are widely used as liquid crystals [1,2], natural and synthetic compounds with various biological activities (insecticidal activity, plant growth stimulators) [3,4,5,6] (Figure 1), ligands for preparing coordination polymers [7], and polyvinyl chloride stabilizers [8]. The reactivity of the azoxy group allows them to be used as building blocks in fine organic synthesis [9,10,11].
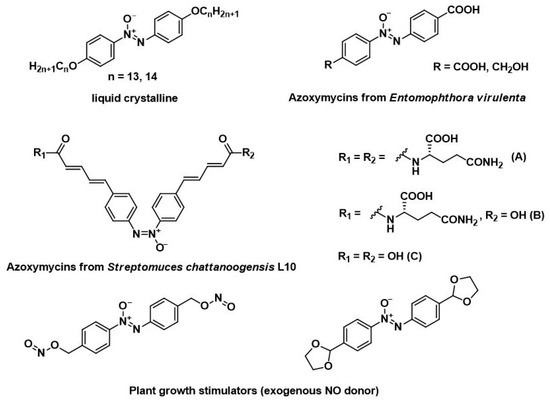
Figure 1.
Examples of liquid crystalline and bioactive natural and synthetic azoxybenzenes.
The main methods for synthesizing azoxybenzenes are the oxidation of aromatic amines [12,13] and azo compounds [14], and the reduction of nitroso compounds [15]. The reduction of nitro compounds is the most widely used method. The classic version uses sodium arsenite [16], sodium alkoxides [17], alkali metal borohydrides [18,19,20], Zn-BiCl3 [21], and Zn/NH4Cl in a mixture with 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim][BF4]) and water [22], or selective catalytic hydrogenation [23,24]. This method cannot be employed for substrates containing other functional groups that are sensitive to reduction. For instance, when reducing nitroaromatic aldehydes or ketones, the carbonyl group is simultaneously reduced [25,26,27,28]. In addition, the reaction is often accompanied by the formation of significant amounts of azo compounds, which complicates the isolation of pure azoxy compounds. In this case, azoxybenzenes with carbonyl groups can be used as starting compounds for the synthesis of analogs of natural and synthetic azoxymycins and other practically useful compounds, such as cyclic acetal prepared from 1,2-bis(4-formylphenyl)diazenoxide and ethylene glycol, which are able to stimulate the growth of grain crops [6].
We are interested in synthesizing 1,2-bis(4-(1,3-dioxolan-2-yl)phenyl)diazene oxide by reducing 2-(4-nitrophenyl)-1,3-dioxolane, using glucose as an eco-friendly reductant in alkaline medium. The reduction of nitro compounds under the action of glucose has been previously described [29]; however, despite its simplicity, the method is not widely used. In addition, in some cases, depending on the conditions of the reduction, the reaction products can be both aromatic amines [30] and azo compounds [31]. The reduction of 2-(4-nitrophenyl)-1,3-dioxolane under Li[AlH4] is accompanied by the formation of azo compounds [32].
2. Results and Discussion
To begin, 2-(4-nitrophenyl)-1,3-dioxolane 3 was synthesized from commercial 4-nitrobenzaldehyde 1 via acetalization with ethylene glycol 2 in a toluene medium (Scheme 1) [33]. Its physical constants and spectral data are in agreement with the literature data. [33].
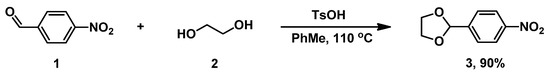
Scheme 1.
Synthesis of dioxolane 3.
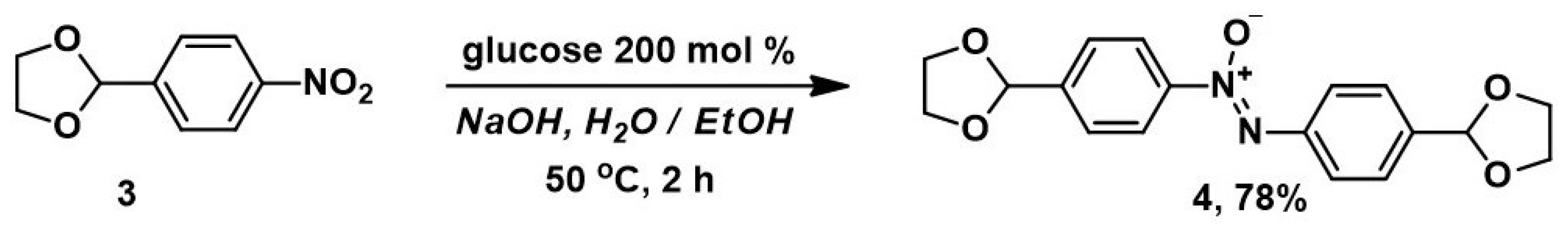
The reduction of 2-(4-nitrophenyl)-1,3-dioxolane 3 was carried out by mixing it with ethanol in a 30% NaOH solution at 50 °C, along with a solution of 200 mol% glucose. Monitoring of the reaction by analytical thin-layer chromatography (TLC) showed that complete conversion is achieved after 2 h of stirring the reaction mixture at 50 °C (Scheme 2).
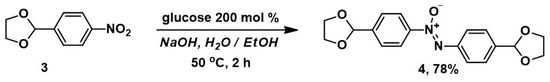
Scheme 2.
Synthesis of azoxybenzene 4 from 2-(4-nitrophenyl)-1,3-dioxolane 3.
We found that conducting experiments in a water–ethanol medium is optimal for carrying out the reduction, since it provides the highest yield of the desired product 4 and minimizes undesirable reactions and the resinification of the reaction mixture. Exchange of ethanol for iso-propanol or tetrahydrofuran leads to the partial destruction of the starting compound 3; it thus incompletely converts, and the yield of the desired product is sharply reduced. When the reaction is carried out at the boiling point, the target compound is contaminated with resinous impurities that are difficult to separate. For complete conversion of the starting compound at room temperature, a time of more than 36 h is necessary; therefore, the reaction was carried out at 50 °C.
The target azoxybenzene 4 was purified by recrystallization from ethanol.
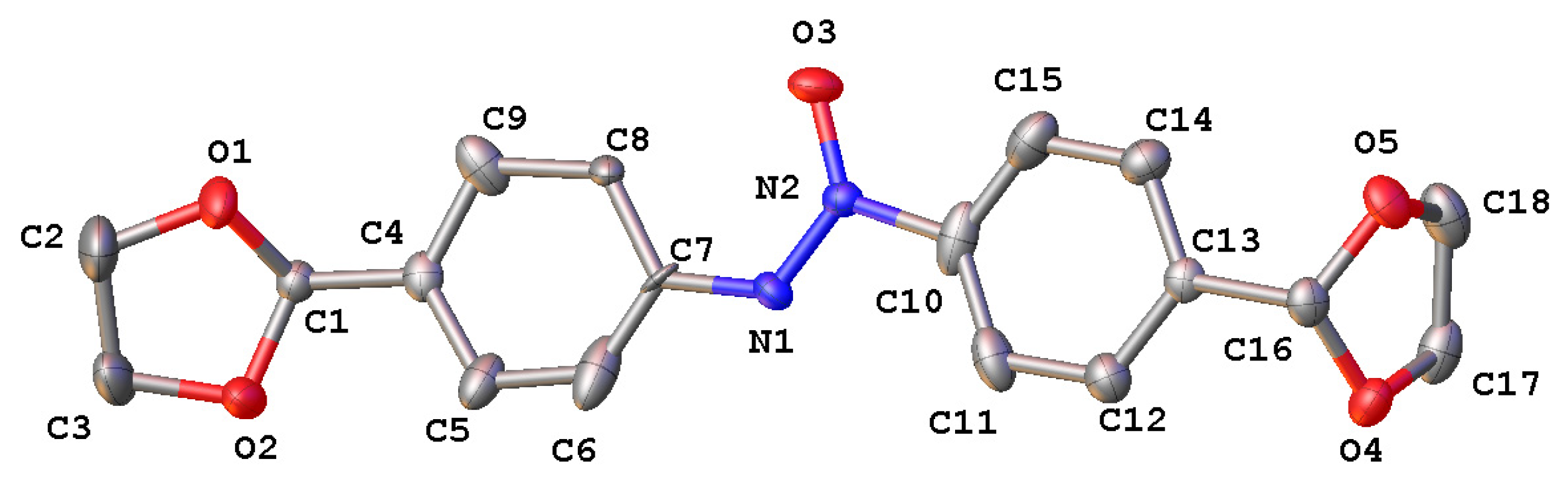
The structure of 1,2-bis(4-(1,3-dioxolan-2-yl)phenyl)diazene oxide 4 was unambiguously confirmed by single-crystal X-ray analysis (Figure 2).
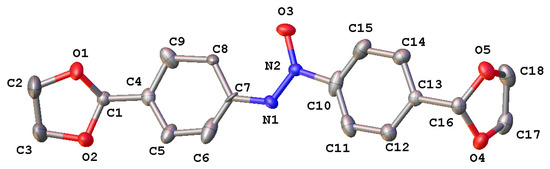
Figure 2.
Crystal structure of compound 4 with labeling schemes and 50% thermal ellipsoids.
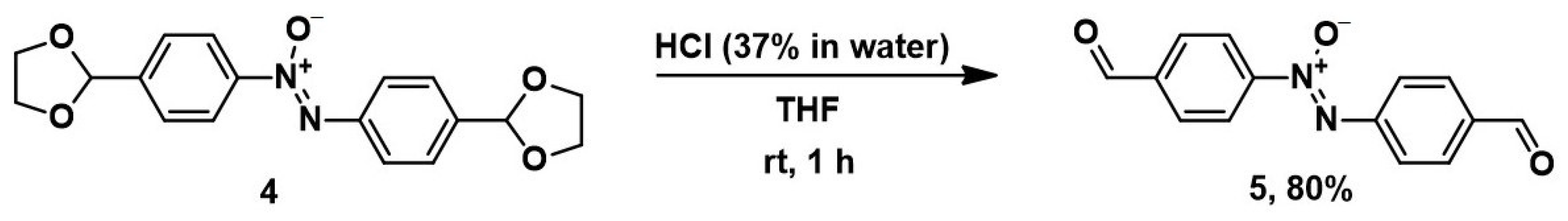
Deprotection of 1,3-dioxolane 4 was carried out with concentrated hydrochloric acid on a solution of 4 in tetrahydrofuran (THF) at room temperature (Scheme 3) [34]. The yield of bis-aldehyde 5 was 80% after recrystallization. Its structure was confirmed by 1H and 13C NMR and mass spectrometry.
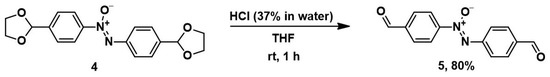
Scheme 3.
Deprotection of 1,3-dioxolane 4 resulting in bis-aldehyde 5.
In summary, using glucose as an eco-friendly reagent allows for the selective reduction of 2-(4-nitrophenyl)-1,3-dioxolane 3 to the azoxy compound 4 with a high yield.
3. Materials and Methods
The reactions were monitored by thin-layer chromatography (Sorbfil, Imid Ltd., Krasnodar, Russia). The 1H-NMR and 13C-NMR spectra were acquired on ECA400 (JEOL) (400 and 100 MHz, respectively) spectrometers in CDCl3 or (CD3)2SO at room temperature (Figures S1–S6). The chemical shifts δ were measured in ppm with reference to the residual solvent resonances (1H: CDCl3, δ = 7.25 ppm; 13C: CDCl3, δ = 77.2 ppm; 1H: (CD3)2SO, δ = 2.49 ppm; 13C: (CD3)2SO, δ = 39.5 ppm). The splitting patterns are referred to as s, singlet; d, doublet; t, triplet; m, multiplet. Coupling constants (J) are given in hertz. IR spectra were recorded on an IR Prestige (Shimadzu, Kyoto, Japan), using tablets of samples with KBr. High-resolution and accurate mass measurements were carried out using a Bruker MaXis Impact (electrospray ionization/time of flight). Mass spectra were recorded on a GCMS−QP2010 Plus (Shimadzu) via electron ionization (70 eV, ionization chamber temperature 250 °C). The melting points were determined on a Stuart SMP30 apparatus and left uncorrected. The commercial reagents employed in the synthesis were 4-Nitrobenzaldehyde (for synthesis, ≥98.0%, Aldrich, St. Louis, MO, USA), Ethylene glycol (99%, ABCR), and D-(+)-Glucose monohydrate (≥99.0%, Aldrich). CCDC 2,080,783 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge at http://www.ccdc.cam.ac.uk/or (accessed date 28 April 2021) from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk.
3.1. 1,2-Bis(4-(1,3-dioxolan-2-yl)phenyl)diazene Oxide (4)
To 6 mL of ethanol, 7.5 mL of a 30% aqueous solution of sodium hydroxide and 0.5 g (2.56 mmol) of 2-(4-nitrophenyl)-1,3-dioxolane 3 were added. The reaction mixture was maintained at 50 °C, and a solution of 1 g (5.12 mmol) of glucose monohydrate in 1 mL of water was added, which was then stirred for 2 h at the specified temperature. Then, the reaction mixture was cooled and diluted with 50 mL of 2M hydrochloric acid, and the formed precipitate was filtered and washed on the filter with distilled water. The resulting residue was purified via recrystallization from EtOH, yielding azoxybenzene 4. Yield 0.34 g (78%); yellowish solid; mp 117–118 °C. IR (KBr): ν = 3130, 3107, 3068 (Csp2-H), 2958, 2893, 2736 (Csp3-H), 1600, 1494 (Csp2-Csp2), 1467 (as N=N(O)), 1384 (sy N=N(O)) cm−1. 1H NMR (CDCl3, 399.78 MHz): δ = 4.02–4.17 (m, 8H, CH2), 5.86 (s, 1H, CH), 5.89 (s, 1H, CH), 7.57–7.63 (m, 4H, CH), 8.16–8.20 (m, 2H, CH), 8.29–8.33 (m, 2H, CH). 13C NMR (CDCl3, 100.5 MHz): δ = 65.35 (CH2), 65.40 (CH2), 102.7 (CH), 103.2 (CH), 122.4 (CH, Ar), 125.6 (CH, Ar), 126.8 (CH, Ar), 127.0 (CH, Ar), 139.3 (C, Ar), 141.8 (C, Ar), 144.5 (C, Ar), 148.7 (C, Ar). HRMS ESI TOF: m/z = 343, 1293 [M+H]+ (343, 1289 calcd for C18H18N2O5). Crystal data for C18H18N2O5 (M = 342.34 g/mol): monoclinic, space group P21/c, a = 4.5333(7) Å, b = 21.946(4) Å, c = 15.968(3) Å, α = 90°, β = 95.627(4)°, γ = 90°, V = 1581.0(4) Å3, Z = 4, T = 120 K, μ = 1.06 cm−1, Dcalc = 1.438 g/cm3. In total, 17,093 reflections were measured, 4876 of which were unique and used in all calculations. The final R1 was 0.0636, and the wR2 was 0.1635 (all data).
3.2. 1,2-Bis(4-formylphenyl)diazenoxide (5)
A solution was made of 0.5 g (1.46 mmol) of 1,2-bis(4-(1,3-dioxolan-2-yl)phenyl)diazen oxide 4 in 10 mL of tetrahydrofuran with 0.5 mL concentrated hydrochloric acid (37% in water). The reaction mixture was stirred for 1 h at room temperature. During this time, according to TLC (hexane/EtOAc, 8:4), complete conversion occurred 4. The reaction mixture was diluted with 50 mL of water, and the formed precipitate was then filtered off and washed on a filter with water. The solid precipitate was recrystallized from the EtOH/EtOAc mixture. Yield 0.29 g (80%); yellowish solid; mp 188–190 °C (dec.) lit. [35] mp 178–180 °C (dec.). IR (KBr): ν = 3107 (Csp2-H), 2850, 2789, 2742 (Csp3-H), 1703, 1687 (C=O), 1597 (Csp2-Csp2), 1463 (as N=N(O)), 1390 (sy N=N(O)) cm−1.1H NMR ((CD3)2SO, 399.78 MHz): δ = 8.05–8.08 (m, 2H, CH), 8.13–8.18 (m, 4H, CH), 8.43–8.47 (m, 2H, CH), 10.06 (s, 1H), 10.16 (s, 1H). 13C NMR ((CD3)2SO, 100.5 MHz): δ = 123.2 (CH, Ar), 125.4 (CH, Ar), 130.2 (CH, Ar), 130.5 (CH, Ar), 136.1 (C, Ar), 138.5 (C, Ar), 147.5 (C, Ar), 150.8 (C, Ar), 192.2 (C=O), 192.4 (C=O). MS (EI, 70 eV, Irel, %): m/z = 254 (17) [M+], 226 (10), 169 (8), 133 (31), 105 (100).
Supplementary Materials
Figure S1: 1H-NMR spectrum of 4; Figure S2: 13C-NMR spectrum of 4; Figure S3: HRMS of 4; Figure S4: IR spectrum of 4; Figure S5.1H-NMR spectrum of 5; Figure S6: 13C-NMR spectrum of 5.
Author Contributions
Conceptualization, V.V.K.; methodology, V.V.K.; software, D.N.K.; validation, V.V.K. and D.N.K.; formal analysis, D.N.K.; investigation, E.S.S. and I.A.L.; resources, E.S.S. and I.A.L.; data curation, V.V.K.; writing—original draft preparation, V.V.K. and D.N.K.; writing—review and editing, V.V.K. and D.N.K.; supervision, V.V.K.; project administration, V.V.K.; funding acquisition, V.V.K., D.N.K., E.S.S. and I.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out with the financial support of the Kuban Science Foundation in the framework of the scientific project № MFI-20.1-28/20.
Data Availability Statement
No applicable.
Acknowledgments
The X-ray diffraction study was performed using the equipment (Bruker APEX DUO diffractometer) of the Center for Molecular Composition Studies of INEOS RAS, and the HRMS study was accomplished with the use of scientific equipment of the Collective Employment Centre «Ecoanalytical Centre», Kuban State University.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Das, P.; Narayan Biswas, A.; Choudhury, A.; Bandyopadhyay, P.; Haldar, S.; Mandal, P.K.; Upreti, S. Novel synthetic route to liquid crystalline 4,4′-bis(n-alkoxy) azoxybenzenes: Spectral characterisation, mesogenicbehaviour and crystal structure of two new members. Liq. Cryst. 2008, 35, 541–548. [Google Scholar] [CrossRef]
- Folcia, C.L.; Alonso, I.; Ortega, J.; Etxebarria, J.; Pintre, I.; Ros, M.B. Achiral bent-core liquid crystals with azo and azoxy linkages: Structural and nonlinear optical properties and photoisomerization. Chem. Mater. 2006, 18, 4617–4626. [Google Scholar] [CrossRef]
- Wibowo, M.; Ding, L. Chemistry and biology of natural azoxy compounds. J. Nat. Prod. 2020, 83, 3482–3491. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-Y.; Li, H.; Zhou, Z.-X.; Mao, X.-M.; Tang, Y.; Chen, X.; Jiang, X.-H.; Liu, Y.; Jiang, H.; Li, Y.-Q. Identification and biosynthetic characterization of natural aromatic azoxy products from Streptomyces Chattanoogensis L10. Org. Lett. 2015, 17, 6114–6117. [Google Scholar] [CrossRef] [PubMed]
- Claydon, N.; Grove, J.F. Metabolic products of Entomophthora Virulenta. J. Chem. Soc. Perkin Trans. 1 1978, 171–173. [Google Scholar] [CrossRef]
- Kotova, V.A.; Rubanova, E.V.; Jatsynin, V.G. Winter Wheat and Barley Roots Stimulant Fertilizer. RU Patent 2,368,140, 7 April 2008. [Google Scholar]
- Wang, J.; Daiguebonne, C.; Suffren, Y.; Roisnel, T.; Freslon, S.; Calvez, G.; Bernot, K.; Guillou, O. A new family of lanthanide-based coordination polymers with azoxybenzene-3,3′,5,5′-tetracarboxylic acid as ligand. Inorg. Chim. Acta. 2019, 488, 208–213. [Google Scholar] [CrossRef]
- Gavrilova, A.O.; Potemkina, O.V.; Kuvshinova, S.A.; Kuznetsov, V.B.; Koifman, O.I. Anisotropic organic azo-and azoxybenzenes exhibiting the properties of light and heat stabilisers of polyvinyl chloride. Int. Polym. Sci. Technol. 2014, 41, 41–42. [Google Scholar] [CrossRef]
- Ichiro, S.; Shigeru, O. The Wallach rearrangement of some 4,4′-disubstituted azoxybenzenes. Bull. Chem. Soc. Jpn. 1983, 56, 643–644. [Google Scholar] [CrossRef]
- Szarmach, M.; Wagner-Wysiecka, E.; Luboch, E. Rearrangement of azoxybenzocrowns into chromophoric hydroxyazobenzocrowns and the use of hydroxyazobenzocrowns for the synthesis of ionophoric biscrown compounds. Tetrahedron 2013, 69, 10893–10905. [Google Scholar] [CrossRef]
- Long, Z.; Wang, Z.; Zhou, D.; Wan, D.; You, J. Rh(III)-catalyzed regio- and chemoselective [4 + 1]-annulation of azoxy compounds with diazoesters for the synthesis of 2H-indazoles: Roles of the azoxy oxygen atom. Org. Lett. 2017, 19, 2777–2780. [Google Scholar] [CrossRef]
- Han, S.; Cheng, Y.; Liu, S.; Tao, C.; Wang, A.; Wei, W.; Yu, H.; Wei, Y. Selective oxidation of anilines to azobenzenes and azoxybenzenes by a molecular Mo oxide catalyst. Angew. Chem. Int. Ed. 2021, 60, 6382–6385. [Google Scholar] [CrossRef]
- Singh, B.; Mandelli, D.; Pescarmona, P.P. Efficient and selective oxidation of aromatic amines to azoxy derivatives over aluminium and gallium oxide catalysts with nanorod morphology. Chem. Cat. Chem. 2020, 12, 593–601. [Google Scholar] [CrossRef]
- Badger, G.M.; Buttery, R.G.; Lewis, G.E. Aromatic azo-compounds. Part I. Oxidation of cis- and trans-azobenzene. J. Chem. Soc. (Resumed) 1953, 2143–2147. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Chen, J.; Lin, L.-J.; Chuang, G.J. Synthesis of azoxybenzenes by reductive dimerization of nitrosobenzene. J. Org. Chem. 2017, 82, 11626–11630. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, H.E.; Palmer, A. Azoxybenzene. Org. Synth. 1943, 2, 57. [Google Scholar] [CrossRef]
- Suter, C.M.; Dains, F.B. The redaction of aromatic nitro compounds with sodium alcoholates. J. Am. Chem. Soc. 1928, 50, 2733–2739. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, B.; Guo, A.; Dong, Z.; Jin, S.; Lu, Y. Reduction of nitroarenes to azoxybenzenes by potassium borohydride in water. Molecules 2011, 16, 3563–3568. [Google Scholar] [CrossRef]
- Ren, P.D.; Pan, S.F.; Dong, T.W.; Wu, S.H. Catalytic reduction of nitroarenes to azoxybenzenes with sodium borohydride in the presence of bismuth. Synth. Commun. 1996, 26, 3903–3908. [Google Scholar] [CrossRef]
- Weill, C.E.; Panson, G.S. The reduction of nitrobenzene to azoxybenzene by sodium borohydride. J. Org. Chem. 1956, 21, 803. [Google Scholar] [CrossRef]
- Borah, H.N.; Dipak Prajapati, D.; Sandhu, J.S.; Ghosh, A.C. Bismuth(III) chloride-zinc promoted selective reduction of aromatic nitro compounds to azoxy compounds. Tetrahedron Lett. 1994, 35, 3167–3170. [Google Scholar] [CrossRef]
- Khan, F.A.; Sudheer, C.H. Oxygen as moderator in the zinc-mediated reduction of aromatic nitro to azoxy compounds. Tetrahedron Lett. 2009, 50, 3394–3396. [Google Scholar] [CrossRef]
- Naveenkumar, A.; Kuruva, P.; Shivakumara, C.; Srilakshmi, C. Mixture of Fuels Approach for the Synthesis of SrFeO3−δ Nanocatalyst and Its Impact on the Catalytic Reduction of Nitrobenzene. Inorg. Chem. 2014, 53, 12178–12185. [Google Scholar] [CrossRef]
- Shukla, A.; Singha, R.K.; Sasaki, T.; Adak, S.; Bhandari, S.; Prasad, V.V.D.N.; Bordoloi, A.; Bal, R. Room temperature selective reduction of nitroarenes to azoxy compounds over Ni-TiO2 catalyst. Mol. Catal. 2020, 490, 110943. [Google Scholar] [CrossRef]
- Shine, H.J.; Mallory, H.E. The reduction of aromatic nitro compounds by potassium borohydride. J. Org. Chem. 1962, 27, 2390–2391. [Google Scholar] [CrossRef]
- Ohe, K.; Takahashi, H.; Uemura, S.; Sugita, N. Sodium benzenetellurolate-catalysed selective reduction of aromatic nitro compounds to azoxy compounds. J. Chem. Soc., Chem. Commun. 1988, 9, 591–592. [Google Scholar] [CrossRef]
- Lakshminarayana, B.; Manna, A.K.; Satyanarayana, G.; Subrahmanyam, C. Palladium nanoparticles on silica nanospheres for switchable reductive coupling of nitroarenes. Catal. Lett. 2020, 150, 2309–2321. [Google Scholar] [CrossRef]
- Bhosale, S.M.; Momin, A.A.; Kunjir, S.; Rajamohanan, P.R.; Kusurkar, R.S. Unexpected observations during the total synthesis of calothrixin B-sodium methoxide as a source of hydride. Tetrahedron Lett. 2014, 55, 155–162. [Google Scholar] [CrossRef]
- Galbraith, H.W.; Degering, E.F.; Hitch, E.F. The alkaline reduction of aromatic nitro compounds with glucose. J. Am. Chem. Soc. 1951, 73, 1323–1324. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, U.; Sharma, S.; Kumar, V.; Singh, B.; Kumar, N. Catalyst-free water mediated reduction of nitroarenes using glucose as a hydrogen source. RSC Adv. 2013, 3, 4894–4898. [Google Scholar] [CrossRef]
- Gardner, H.C.; Kennedy, A.R.; McCarney, K.M.; Staunton, E.; Stewart, H.; Teat, S.J. Structures of five salt forms of disulfonated monoazo dyes. Acta Cryst. C Struct. Chem. 2020, 76, 972–981. [Google Scholar] [CrossRef]
- Masciello, L.; Potvin, P.G. One-pot synthesis of terpyridines and macrocyclization to C3-symmetric cyclosexipyridines. Can. J. Chem. 2003, 81, 209–218. [Google Scholar] [CrossRef]
- Frlan, R.; Kovač, A.; Blanot, D.; Gobec, S.; Pečar, S.; Obreza, A. Design and synthesis of novel N-benzylidenesulfonohydrazide inhibitors of MurC and MurD as potential antibacterial agents. Molecules 2008, 13, 11–30. [Google Scholar] [CrossRef]
- Wuts, P.G.M. Greene’s Protective Groups in Organic Synthesis, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–1360. [Google Scholar] [CrossRef]
- Boduszek, B.; Halama, A. Nitrobenzyl (α-amino)phosphonates. part 2[1]. Cleavage of 4-nitrobenzyl(α-amino)phosphonic acids in aqueous sodium hydroxide solution. Phosphorus Sulfur Silicon Relat. Elem. 1998, 141, 239–250. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).