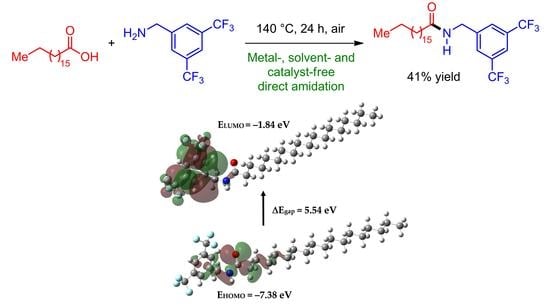
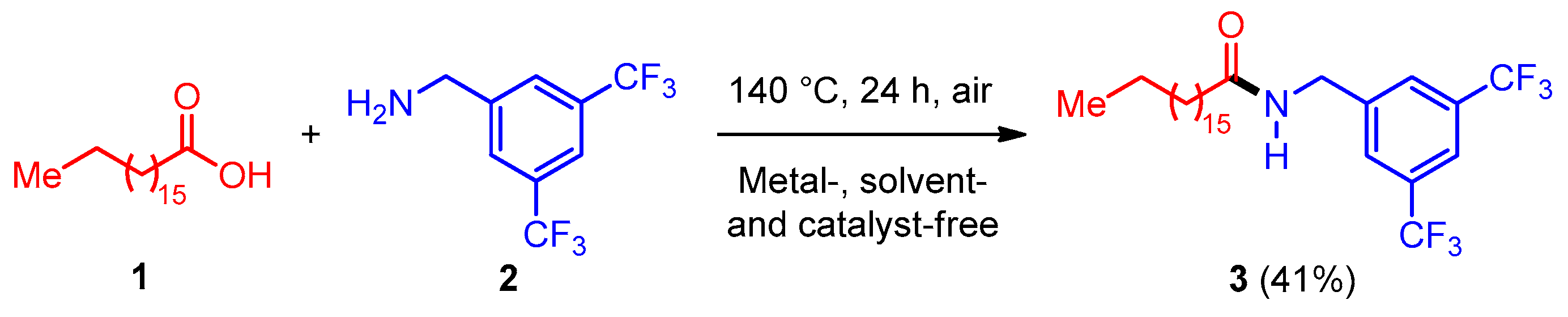
Synthesis, Characterization, and DFT Studies of N-(3,5-Bis(trifluoromethyl)benzyl)stearamide
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
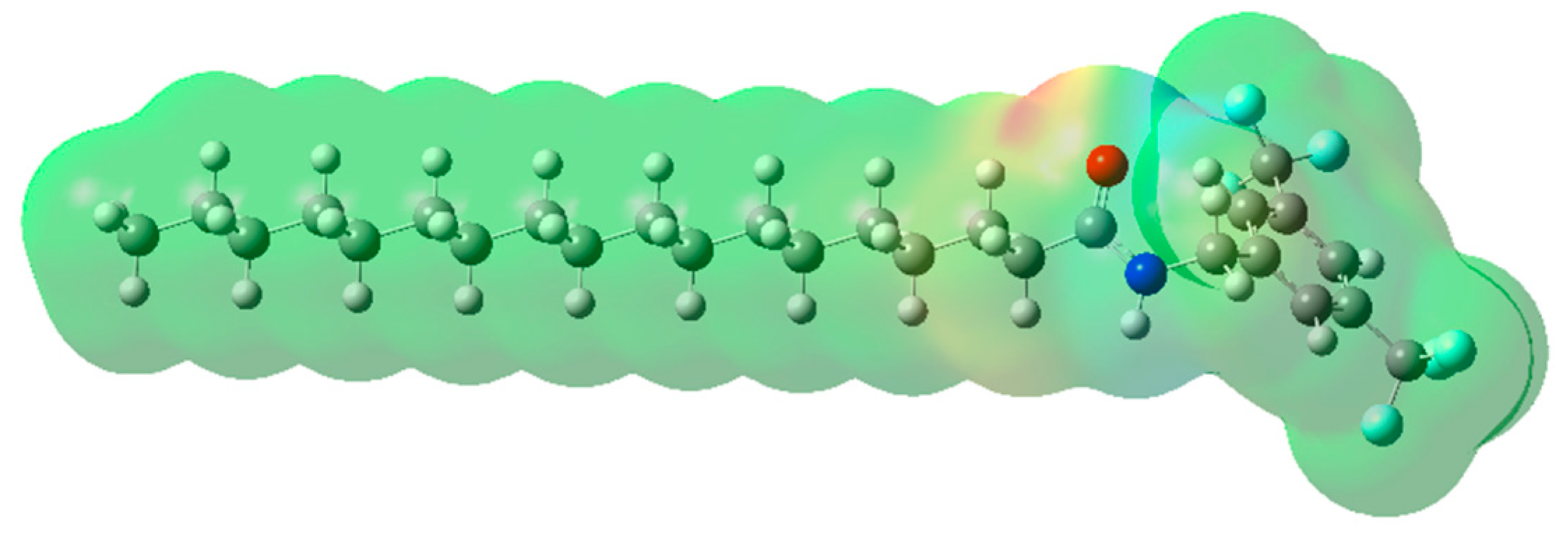
2.2. Molecular Electrostatic Potential (MEP) Mapping
2.3. Frontier Orbitals and Global Reactivity Descriptors
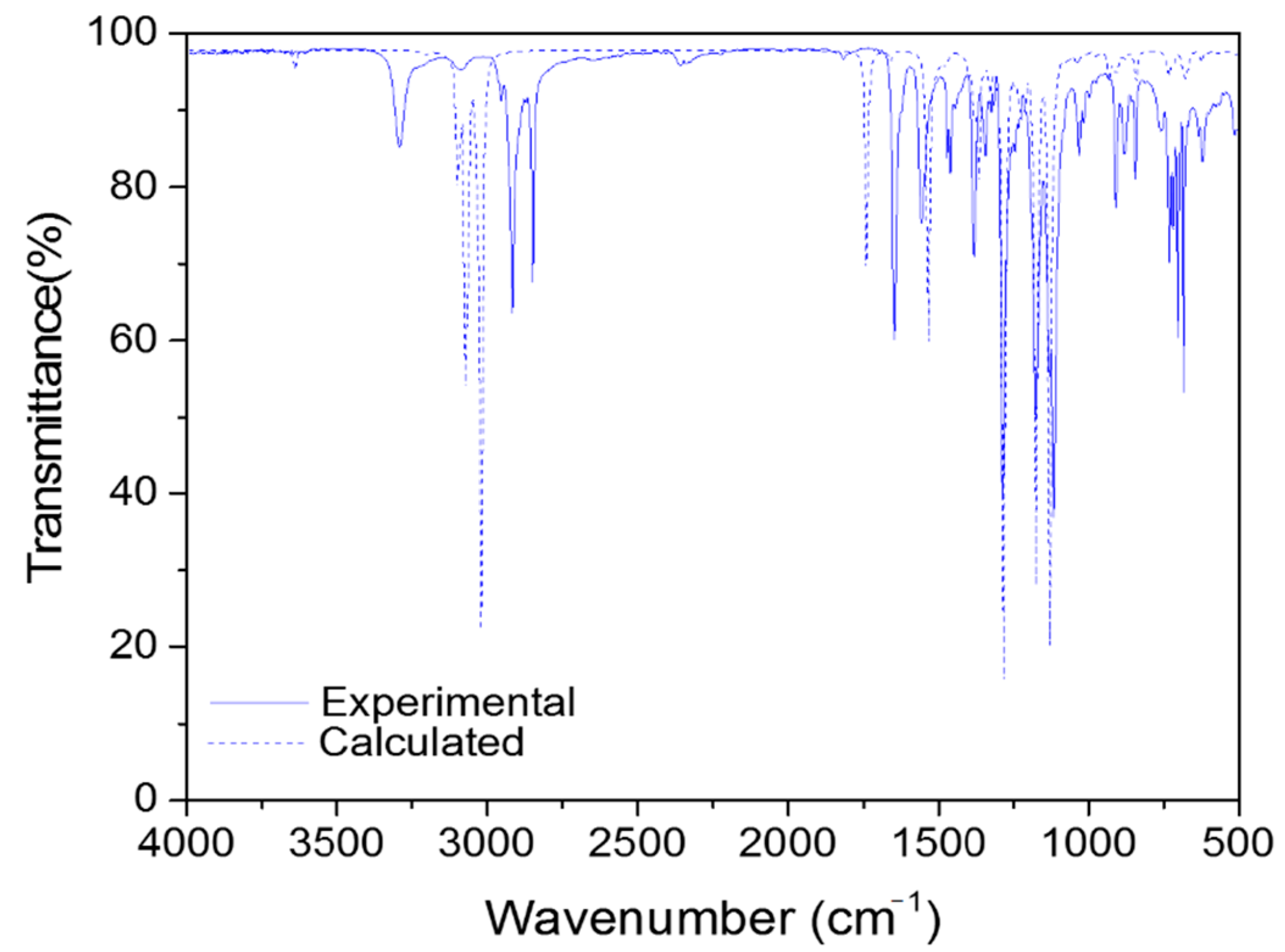
2.4. Vibrational Analysis
3. Materials and Methods
3.1. General Information
3.2. Computational Study
3.3. Synthesis of N-(3,5-Bis(trifluoromethyl)benzyl)stearamide 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pattabiraman, V.; Bode, J. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, S.; Tang, K.-C.; Raj, M. Amide bond activation of biological molecules. Molecules 2018, 23, 2615. [Google Scholar] [CrossRef]
- Dunetz, J.R.; Magano, J.; Weisenburger, G.A. Large-scale applications of amide coupling reagents for the synthesis of pharmaceuticals. Org. Process Res. Dev. 2016, 20, 140–177. [Google Scholar] [CrossRef]
- Bloemendal, V.R.L.J.; Janssen, M.A.C.H.; van Hest, J.C.M.; Rutjes, F.P.J.T. Continuous one-flow multi-step synthesis of active pharmaceutical ingredients. React. Chem. Eng. 2020, 5, 1186–1197. [Google Scholar] [CrossRef]
- Wang, B.-L.; Zhu, H.-W.; Ma, Y.; Xiong, L.-X.; Li, Y.-Q.; Zhao, Y.; Zhang, J.-F.; Chen, Y.-W.; Zhou, S.; Li, Z.-M. Synthesis, insecticidal activities, and SAR studies of novel pyridylpyrazole acid derivatives based on amide bridge modification of anthranilic diamide insecticides. J. Agric. Food Chem. 2013, 61, 5483–5493. [Google Scholar] [CrossRef]
- Pereira, E.; Farias, E.; Ribeiro, A.; Alvarenga, E.; Aguiar, A.; Ferreira, J.; Picanço, M. Toxicity of piperine amide analogs toward the tomato pinworm Tuta absoluta (lepidoptera: Gelechiidae) and risk assessment for two predators. Horticulturae 2019, 5, 70. [Google Scholar] [CrossRef]
- Fayemiwo, K.A.; Chiarasumran, N.; Nabavi, S.A.; Loponov, K.N.; Manović, V.; Benyahia, B.; Vladisavljević, G.T. Eco-friendly fabrication of a highly selective amide-based polymer for CO2 capture. Ind. Eng. Chem. Res. 2019, 58, 18160–18167. [Google Scholar] [CrossRef]
- Simplício, A.L.; Clancy, J.M.; Gilmer, J.F. Prodrugs for amines. Molecules 2008, 13, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.R.; Wilson, I.D.; Gill, R.U.; Butler, P.J.; Dilworth, C.; Athersuch, T.J. Metabolic hydrolysis of aromatic amides in selected rat, minipig, and human in vitro systems. Sci. Rep. 2018, 8, 2405. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide bond bioisosteres: Strategies, synthesis, and successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef] [PubMed]
- Wodtke, R.; Wodtke, J.; Hauser, S.; Laube, M.; Bauer, D.; Rothe, R.; Neuber, C.; Pietsch, M.; Kopka, K.; Pietzsch, J.; et al. Development of an 18F-labeled irreversible inhibitor of transglutaminase 2 as radiometric tool for quantitative expression profiling in cells and tissues. J. Med. Chem. 2021, 64, 3462–3478. [Google Scholar] [CrossRef]
- Gisemba, S.A.; Ferracane, M.J.; Murray, T.F.; Aldrich, J.V. Conformational constraint between aromatic residue side chains in the “message” sequence of the peptide arodyn using ring closing metathesis results in a potent and selective kappa opioid receptor antagonist. J. Med. Chem. 2021, 64, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.S.; Salim, S.D.; Sawant, R.V.; Akamanchi, K.G. Sulfated tungstate: A new solid heterogeneous catalyst for amide synthesis. Green Chem. 2010, 12, 1707–1710. [Google Scholar] [CrossRef]
- Saghatelian, A.; McKinney, M.K.; Bandell, M.; Patapoutian, A.; Cravatt, B.F. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry 2006, 45, 9007–9015. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Reggio, P.H. Endocannabinoid binding to the cannabinoid receptors: What is known and what remains unknown. Curr. Med. Chem. 2010, 17, 1468–1486. [Google Scholar] [CrossRef]
- Huang, S.M.; Bisogno, T.; Petros, T.J.; Chang, S.Y.; Zavitsanos, P.A.; Zipkin, R.E.; Sivakumar, R.; Coop, A.; Maeda, D.Y.; De Petrocellis, L.; et al. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J. Biol. Chem. 2001, 276, 42639–42644. [Google Scholar] [CrossRef]
- Farrell, E.K.; Merkler, D.J. Biosynthesis, degradation, and pharmacological importance of the fatty acid amides. Drug Discov. Today 2008, 13, 558–568. [Google Scholar] [CrossRef]
- Tripathi, R.K.P. A perspective review on fatty acid amide hydrolase (FAAH) inhibitors as potential therapeutic agents. Eur. J. Med. Chem. 2020, 188, 111953. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Sharma, U.; Kumar, N.; Singh, B. Emerging catalytic methods for amide synthesis. Curr. Org. Synth. 2013, 10, 241–264. [Google Scholar] [CrossRef]
- Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. Catalytic amide formation from non-activated carboxylic acids and amines. Chem. Soc. Rev. 2014, 43, 2714–2742. [Google Scholar] [CrossRef] [PubMed]
- Lukasik, N.; Wagner-Wysiecka, E. A review of amide bond formation in microwave organic synthesis. Curr. Org. Synth. 2014, 11, 592–604. [Google Scholar] [CrossRef]
- Wang, X. Challenges and outlook for catalytic direct amidation reactions. Nat. Catal. 2019, 2, 98–102. [Google Scholar] [CrossRef]
- Sabatini, M.T.; Boulton, L.T.; Sneddon, H.F.; Sheppard, T.D. A green chemistry perspective on catalytic amide bond formation. Nat. Catal. 2019, 2, 10–17. [Google Scholar] [CrossRef]
- Reddy, T.N.; de Lima, D.P. Recent advances in the functionalization of hydrocarbons: Synthesis of amides and its derivatives. Asian J. Org. Chem. 2019, 8, 1227–1262. [Google Scholar] [CrossRef]
- Massolo, E.; Pirola, M.; Benaglia, M. Amide bond formation strategies: Latest advances on a dateless transformation. Eur. J. Org. Chem. 2020, 2020, 4641–4651. [Google Scholar] [CrossRef]
- Chaparro, S.; Rojas, H.; Castillo, J.C.; Portilla, J.; Romanelli, G.P.; Pineda, A.; Elsharif, A.M.; Martinez, J.J.; Luque, R. Solventless amide synthesis catalyzed by biogenic CaCO3 materials. ACS Sustain. Chem. Eng. 2020, 8, 13139–13146. [Google Scholar] [CrossRef]
- Becerra, D.; Castillo, J.; Insuasty, B.; Cobo, J.; Glidewell, C. Synthesis of N-substituted 3-(2-aryl-2-oxoethyl)-3-hydroxy indolin-2-ones and their conversion to N-substituted (E)-3-(2-aryl-2-oxoethylidene)indolin-2-ones: Synthetic sequence, spectroscopic characterization and structures of four 3-hydroxy compounds and five oxo ethylidene products. Acta Cryst. 2020, C76, 433–445. [Google Scholar] [CrossRef]
- Becerra, D.; Rojas, H.; Castillo, J.-C. 3-(tert-Butyl)-N-(4-methoxybenzyl)-1-methyl-1H-pyrazol-5-amine. Molbank 2021, 2021, M1196. [Google Scholar] [CrossRef]
- Bianchini, G.; Ribelles, P.; Becerra, D.; Ramos, M.T.; Menéndez, J.C. Efficient synthesis of 2-acylquinolines based on an azavinylogous Povarov reaction. Org. Chem. Front. 2016, 3, 412–422. [Google Scholar] [CrossRef]
- Acosta, P.; Becerra, D.; Goudedranche, S.; Quiroga, J.; Constantieux, T.; Bonne, D.; Rodriguez, R. Exploiting the reactivity of 1,2-ketoamides: Enantioselective synthesis of functionalized pyrrolidines and pyrrolo-1,4-benzodiazepine-2,5-diones. Synlett 2015, 26, 1591–1595. [Google Scholar] [CrossRef]
- Castillo, J.-C.; Orrego-Hernández, J.; Portilla, J. Cs2CO3-Promoted direct N-alkylation: Highly chemoselective synthesis of N-alkylated benzylamines and anilines. Eur. J. Org. Chem. 2016, 2016, 3824–3835. [Google Scholar] [CrossRef]
- Abonia, R.; Castillo, J.C.; Garay, A.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J.; D’Vries, R. A facile synthesis of stable β-amino-N-/O-hemiacetals through a catalyst-free three-component Mannich-type reaction. Tetrahedron Lett. 2017, 58, 1490–1494. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. 1988, 37B, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Glover, S.A.; Rosser, A.A. Heteroatom substitution at amide nitrogen-resonance reduction and HERON reactions of anomeric amides. Molecules 2018, 23, 2834. [Google Scholar] [CrossRef] [PubMed]
- Antonijevic, I.S.; Janjic, G.V.; Milcic, M.; Zaric, S.D. Preferred geometries and energies of sulfur−sulfur interactions in crystal structures. Cryst. Growth Des. 2016, 16, 632–639. [Google Scholar] [CrossRef]
- Nataraj, A.; Balachandran, V.; Karthick, T. Molecular orbital studies (hardness, chemical potential, electrophilicity, and first electron excitation), vibrational investigation and theoretical NBO analysis of 2-hydroxy-5-bromobenzaldehyde by density functional method. J. Mol. Struct. 2013, 1031, 221–233. [Google Scholar] [CrossRef]
- Politzer, P.; Abu-Awwad, F. A comparative analysis of Hartree-Fock and Kohn-Sham orbital energies. Theor. Chem. Acc. 1998, 99, 83–87. [Google Scholar] [CrossRef]
- Parr, R.G.; von Szentpaly, L.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
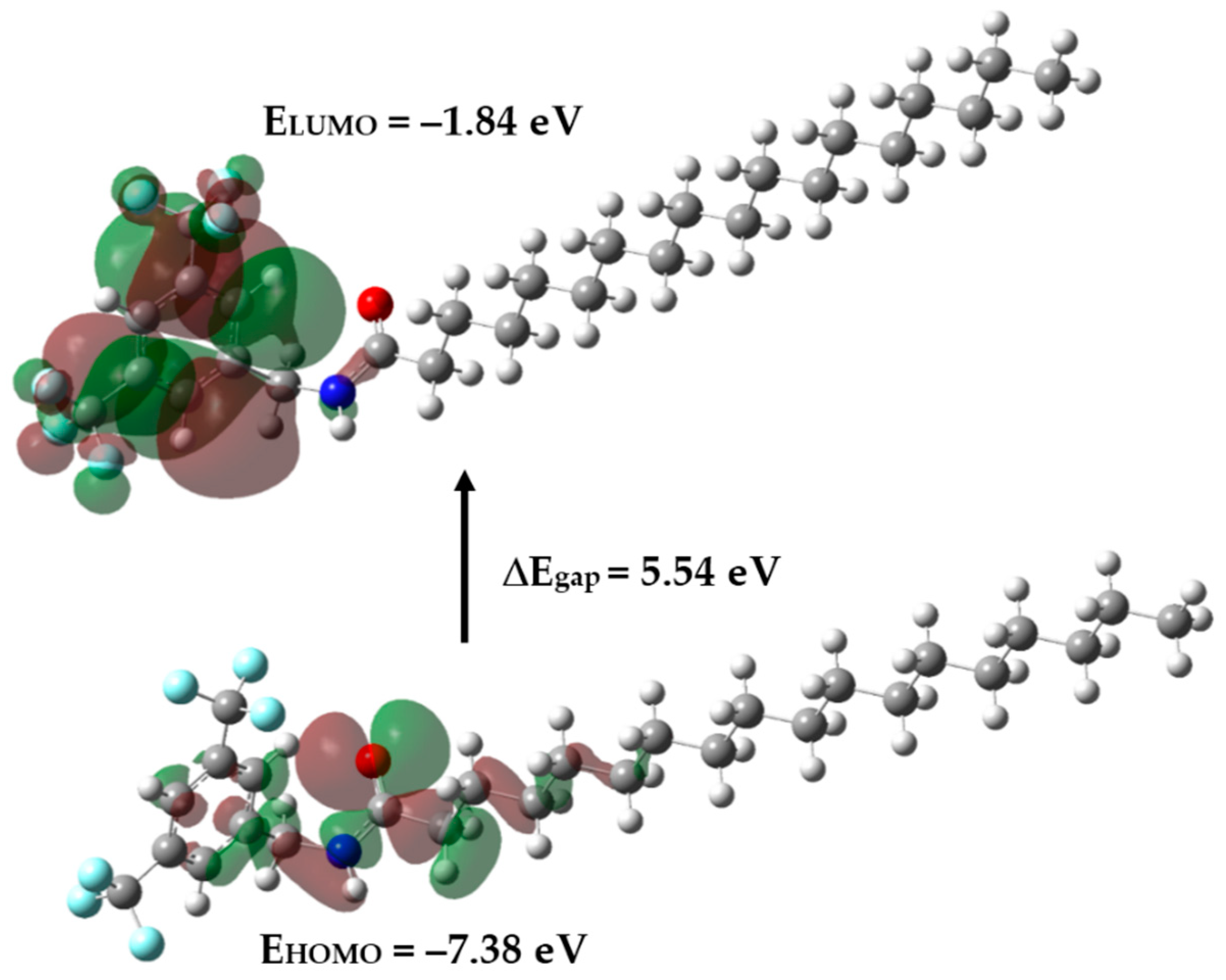
| Parameters | Amide 3 |
|---|---|
| HOMO energy | −7.38 |
| LUMO energy | −1.84 |
| HOMO–LUMO energy gap | 5.54 |
| Ionization potential (IP) | 7.38 |
| Electron affinity (EA) | 1.84 |
| Electrophilicity index (ω) | 1.92 |
| Chemical potential (μ) | −4.61 |
| Electronegativity (χ) | 4.61 |
| Hardness (η) | 5.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas-Torres, A.; Rojas, H.; Martínez, J.J.; Becerra, D.; Castillo, J.-C. Synthesis, Characterization, and DFT Studies of N-(3,5-Bis(trifluoromethyl)benzyl)stearamide. Molbank 2021, 2021, M1215. https://doi.org/10.3390/M1215
Salinas-Torres A, Rojas H, Martínez JJ, Becerra D, Castillo J-C. Synthesis, Characterization, and DFT Studies of N-(3,5-Bis(trifluoromethyl)benzyl)stearamide. Molbank. 2021; 2021(2):M1215. https://doi.org/10.3390/M1215
Chicago/Turabian StyleSalinas-Torres, Angélica, Hugo Rojas, José J. Martínez, Diana Becerra, and Juan-Carlos Castillo. 2021. "Synthesis, Characterization, and DFT Studies of N-(3,5-Bis(trifluoromethyl)benzyl)stearamide" Molbank 2021, no. 2: M1215. https://doi.org/10.3390/M1215
APA StyleSalinas-Torres, A., Rojas, H., Martínez, J. J., Becerra, D., & Castillo, J.-C. (2021). Synthesis, Characterization, and DFT Studies of N-(3,5-Bis(trifluoromethyl)benzyl)stearamide. Molbank, 2021(2), M1215. https://doi.org/10.3390/M1215