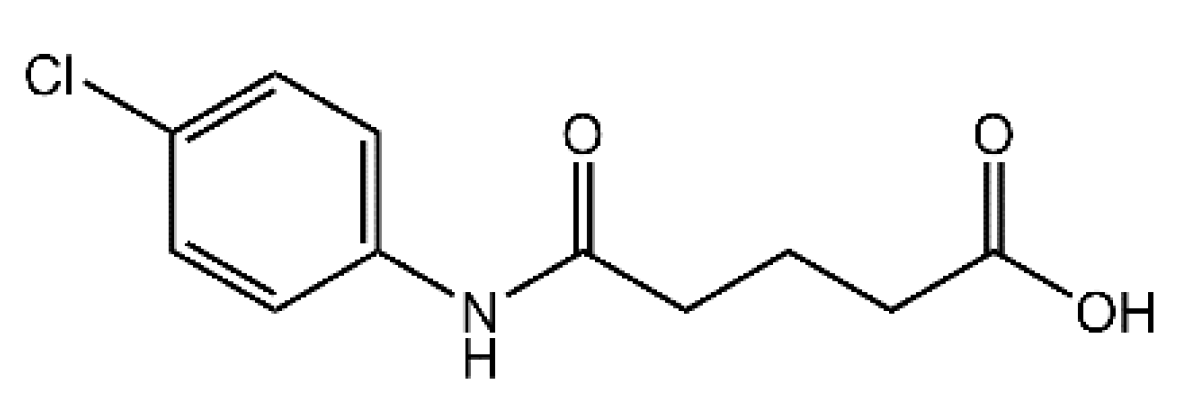
4-[(4-Chlorophenyl)carbamoyl]butanoic Acid
Abstract
1. Introduction
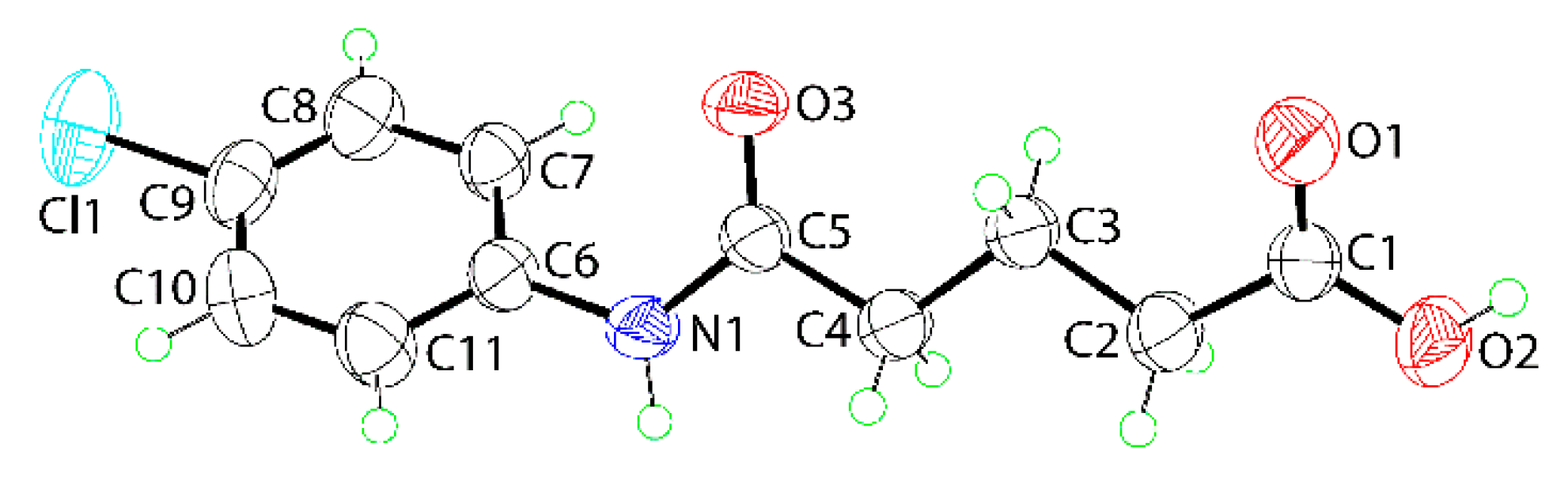
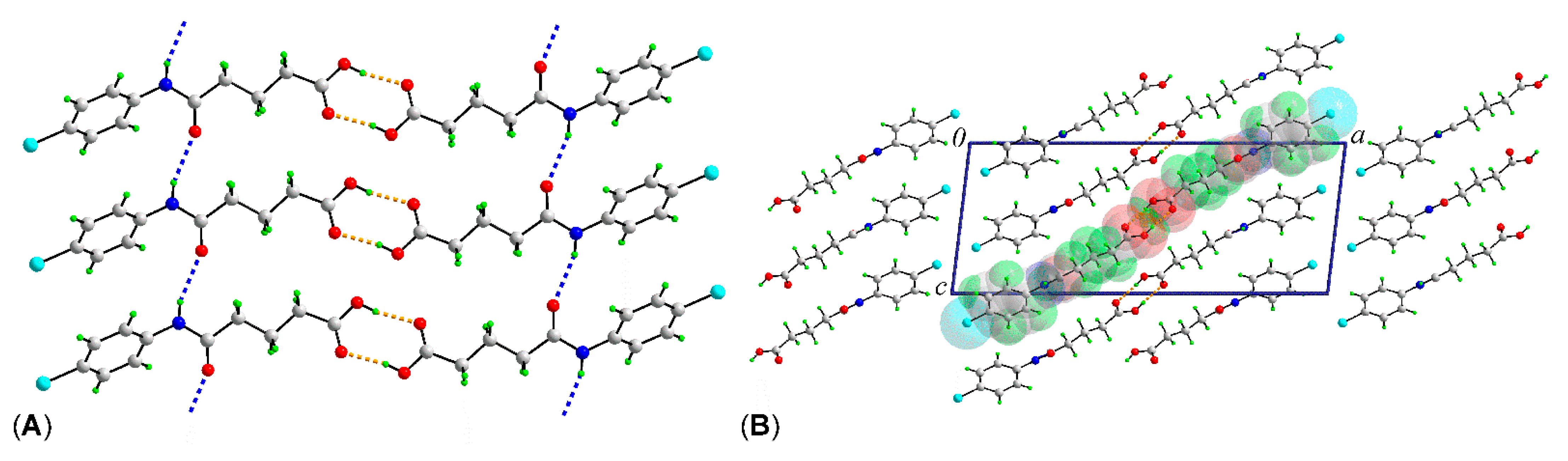
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization of (1)
3.3. Crystallography
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanifa, B.; Sirajuddin, M.; Lo, K.M.; Tiekink, E.R.T. Crystal structure of 4-[(4-methoxy-2-nitrophenyl)carbamoyl]butanoic acid, C12H14N2O6. Z. Kristallogr. New Cryst. Struct. 2020, 235, 1435–1437. [Google Scholar] [CrossRef]
- Hanifa, B.; Sirajuddin, M.; Lo, K.M.; Tiekink, E.R.T. Crystal structure of 4-[(2-methoxyphenyl)carbamoyl]butanoic acid, C12H15NO4. Z. Kristallogr. New Cryst. Struct. 2020, 235, 1481–1483. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Hanifa, B.; Ullah, S.; Lo, K.M.; Tiekink, E.R.T. Crystal structure of 4-[(3,5-dichlorophenyl)carbamoyl]butanoic acid, C11H11Cl2NO3. Z. Kristallogr. New Cryst. Struct. 2020, 235, 1495–1497. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Hanifa, B.; Ullah, S.; Lo, K.M.; Tiekink, E.R.T. Crystal structure of 4-[(3-methoxyphenyl)carbamoyl]butanoic acid, C12H15NO4. Z. Kristallogr. New Cryst. Struct. 2020, 235, 1519–1521. [Google Scholar] [CrossRef]
- Evans, D.W.S.; Roberts, J.C. Synthesis of potential antibacterial agents. Part III. Derivatives of some αα′-dialkylglutaric acids. J. Chem. Soc. 1957, 2104–2106. [Google Scholar] [CrossRef]
- Larsen, S.P.; Scholes, V.P.; Skinner, C.G. Effect of 4’-chloroglutaranilic acid on growth and development of sunflower seedlings. Am. J. Bot. 1974, 61, 290–295. [Google Scholar] [CrossRef]
- Stiz, D.S.; Souza, M.M.; Golin, V.; Neto, R.A.; Corrêa, R.; Nunes, R.J.; Yunes, R.A.; Cechinel-Filho, V. Antinociceptive properties of N-aryl-glutaramic acids and N-aryl-glutarimides. Pharmazie 2000, 55, 942–944. [Google Scholar] [PubMed]
- Weißenfels, M.; Kaubisch, S. Über umsetzungen von glutarsäureimiden und glutaramidsäuren mit dem Vilsmeier-Haack-reagens und folgereaktionen. Z. Chem. 1982, 22, 23–24. [Google Scholar] [CrossRef]
- Dhivare, R.S.; Rajput, S.S. Microwave synthesis and antimicrobial influence of some novel 2,2′-(N-phenylpiperidine-2,6-diylidene)dimalononitrile compounds derived from n-phenyl glutarimides. Heterocycl. Lett. 2016, 6, 443–451. [Google Scholar] [CrossRef]
- Spek, A.L. checkCIF validation ALERTS: What they mean and how to respond. Acta Crystallogr. E 2020, 76, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlis PRO; Rigaku Corporation: Oxford, UK, 2017. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. DIAMOND; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanifa, B.; Sirajuddin, M.; Bari, A.; Lee, S.M.; Lo, K.M.; Tiekink, E.R.T. 4-[(4-Chlorophenyl)carbamoyl]butanoic Acid. Molbank 2021, 2021, M1209. https://doi.org/10.3390/M1209
Hanifa B, Sirajuddin M, Bari A, Lee SM, Lo KM, Tiekink ERT. 4-[(4-Chlorophenyl)carbamoyl]butanoic Acid. Molbank. 2021; 2021(2):M1209. https://doi.org/10.3390/M1209
Chicago/Turabian StyleHanifa, Bibi, Muhammad Sirajuddin, Ahmed Bari, See Mun Lee, Kong Mun Lo, and Edward R. T. Tiekink. 2021. "4-[(4-Chlorophenyl)carbamoyl]butanoic Acid" Molbank 2021, no. 2: M1209. https://doi.org/10.3390/M1209
APA StyleHanifa, B., Sirajuddin, M., Bari, A., Lee, S. M., Lo, K. M., & Tiekink, E. R. T. (2021). 4-[(4-Chlorophenyl)carbamoyl]butanoic Acid. Molbank, 2021(2), M1209. https://doi.org/10.3390/M1209







