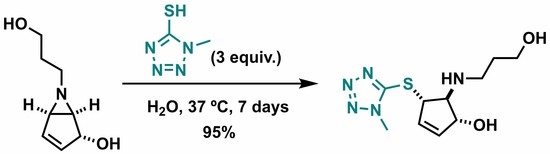
(1R,4S,5S)-5-((3-Hydroxypropyl)amino)-4-((1-methyl-1H-tetrazol-5-yl)thio)cyclopent-2-en-1-ol
Abstract
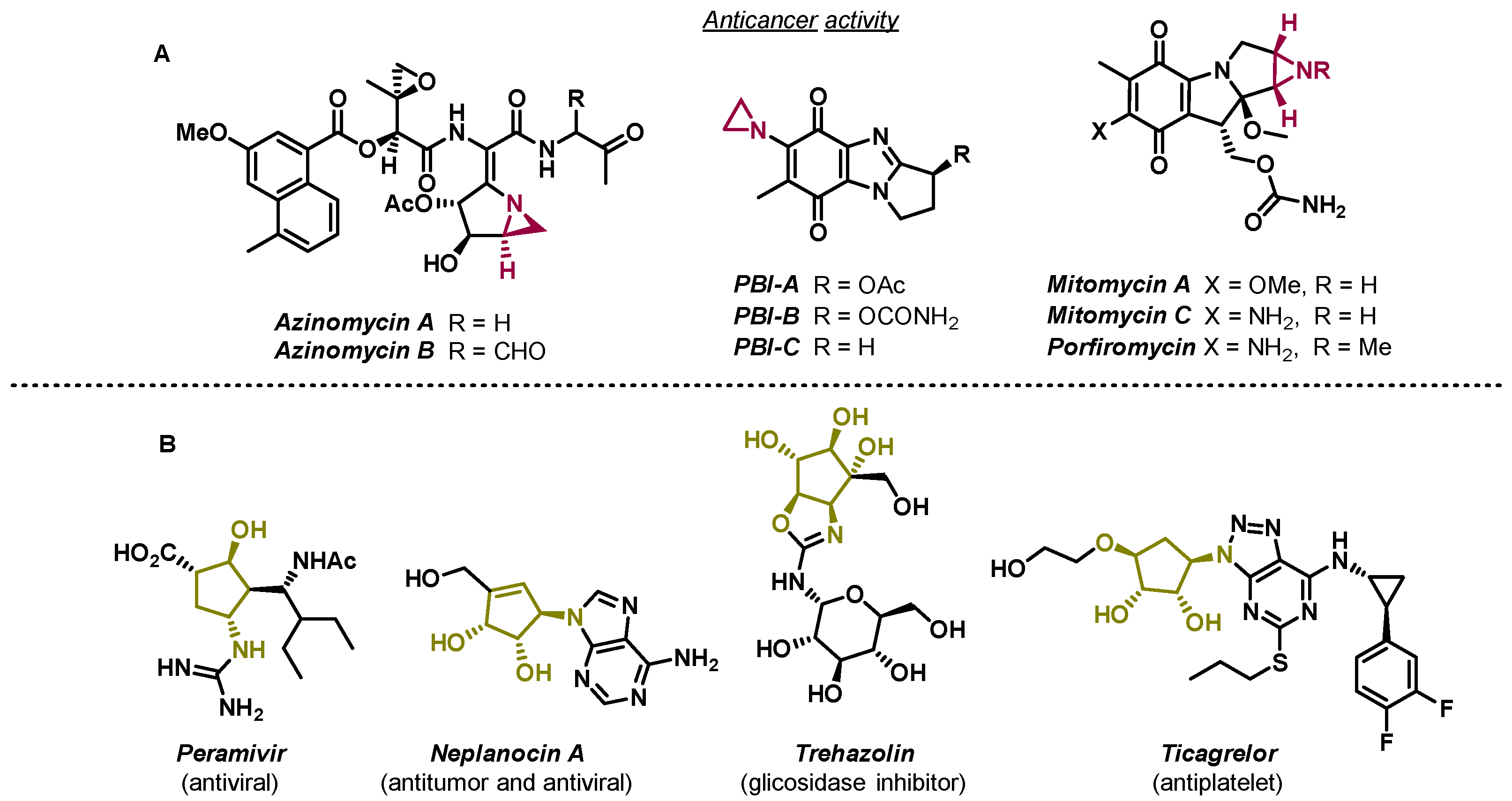
1. Introduction
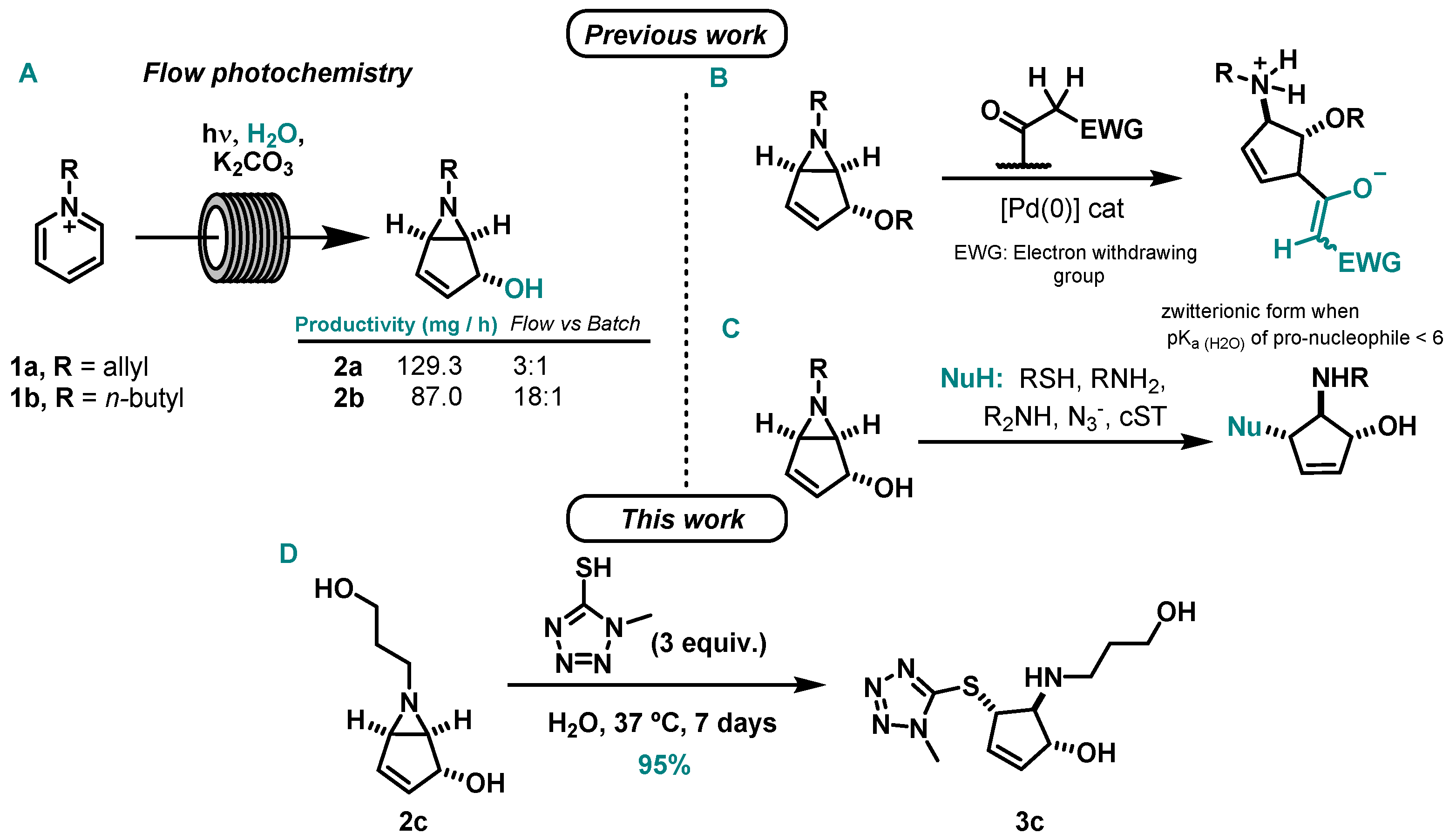
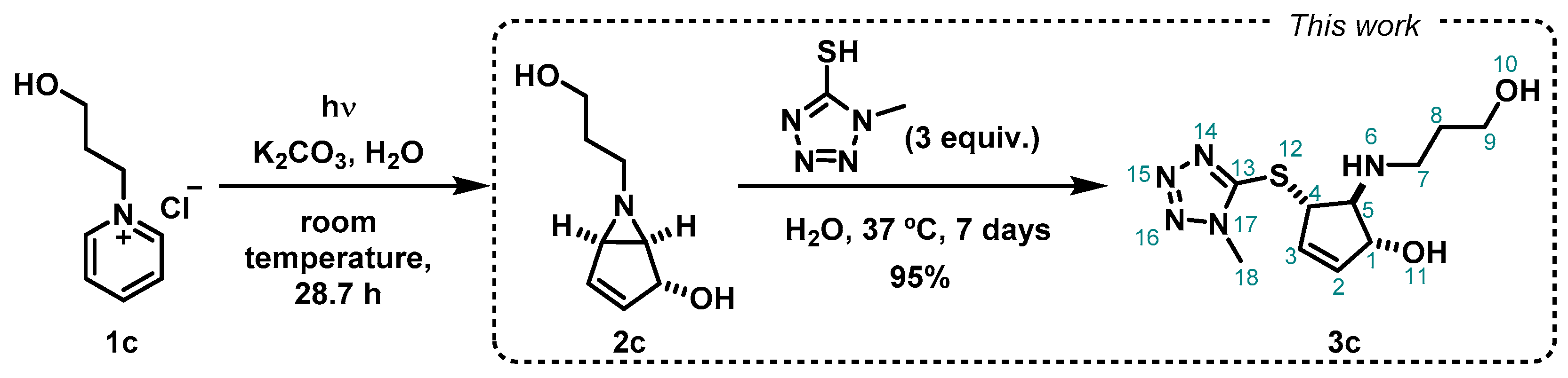
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, Z. Catalytic Asymmetric Transformations of Racemic Aziridines. Synthesis 2020, 52, 1738–1750. [Google Scholar] [CrossRef]
- Sweeney, J.B. Aziridines: Epoxides’ ugly cousins? Chem. Soc. Rev. 2002, 31, 247–258. [Google Scholar] [CrossRef]
- Košak, U.; Hrast, M.; Knez, D.; Maraš, N.; Črnugelj, M.; Gobec, S. Convenient syntheses of orthogonally protected aminocyclopentitols from aldopentoses. Tetrahedron Lett. 2015, 56, 529–531. [Google Scholar] [CrossRef]
- Marin, L.; Force, G.; Gandon, V.; Schulz, E.; Lebœuf, D. Aza-Piancatelli Cyclization as a Platform for the Preparation of Scaffolds of Natural Compounds: Application to the Total Synthesis of Bruceolline D. Eur. J. Org. Chem. 2020, 2020, 5323–5328. [Google Scholar] [CrossRef]
- Kaplan, L.; Pavlik, J.W.; Wilzbach, K.E. Photohydrataion of Pyridinium Ions. J. Am. Chem. Soc. 1972, 94, 3283–3284. [Google Scholar] [CrossRef]
- Delgado, A. Recent Advances in the Chemistry of Aminocyclitols. Eur. J. Org. Chem. 2008, 2008, 3893–3906. [Google Scholar] [CrossRef]
- Diaz, L.; Delgado, A. Medicinal Chemistry of Aminocyclitols. Curr. Med. Chem. 2010, 17, 2393–2418. [Google Scholar] [CrossRef]
- Yoon, U.C.; Quillen, S.L.; Mariano, P.S.; Swanson, R.; Stavinoha, J.L.; Bay, E. Electron transfer initiated photocyclizations of N-allylpridinium and quinolinium salts. Tetrahedron Lett. 1982, 23, 919–922. [Google Scholar] [CrossRef]
- Ling, R.; Mariano, P.S. A Demonstration of the Synthetic Potential of Pyridinium Salt Photochemistry by Its Application to a Stereocontrolled Synthesis of (+)-Mannostatin A 1. J. Org. Chem. 1998, 63, 6072–6076. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Song, L.; Mariano, P.S. A concise sequential photochemical-metathesis approach for the synthesis of (+)-castanospermine and possible uniflorine-A stereoisomers. Tetrahedron 2005, 61, 8888–8894. [Google Scholar] [CrossRef]
- Song, L.; Duesler, E.N.; Mariano, P.S. Stereoselective synthesis of polyhydroxylated indolizidines based on pyridinium salt photochemistry and ring rearrangement metathesis. J. Org. Chem. 2004, 69, 7284–7293. [Google Scholar] [CrossRef] [PubMed]
- Kawatkar, S.P.; Kuntz, D.A.; Woods, R.J.; Rose, D.R.; Boons, G.J. Structural basis of the inhibition of Golgi α-mannosidase II by mannostatin A and the role of the thiomethyl moiety in ligand-protein interactions. J. Am. Chem. Soc. 2006, 128, 8310–8319. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Ling, R.; Kim, A.; Mariano, P.S. A Versatile Approach to the Synthesis of (+) -Mannostatin A Analogues. J. Org. Chem. 2000, 65, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Siopa, F.; António, J.P.M.; Afonso, C.A.M. Flow-Assisted Synthesis of Bicyclic Aziridines via Photochemical Transformation of Pyridinium Salts. Org. Process Res. Dev. 2018, 22, 551–556. [Google Scholar] [CrossRef]
- Fortunato, M.A.G.; Ly, C.-P.; Siopa, F.; Afonso, C.A.M. Process Intesification for the Synthesis of 6-Allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol from 1-Allylpyridinium Salt Using a Continuous UV-Light Photoflow Approach. Methods Protoc. 2019, 2, 67. [Google Scholar] [CrossRef]
- Colombo, C.; Pinto, B.M.; Bernardi, A.; Bennet, A.J. Synthesis and evaluation of influenza A viral neuraminidase candidate inhibitors based on a bicyclo[3.1.0]hexane scaffold. Org. Biomol. Chem. 2016, 14, 6539–6553. [Google Scholar] [CrossRef]
- Vale, J.R.; Siopa, F.; Branco, P.S.; Afonso, C.A.M. Ring Opening of 6-Azabicyclo-[3.1.0]hex-3-en-2-ols in Water under Mild Conditions. Eur. J. Org. Chem. 2016, 2016, 2048–2053. [Google Scholar] [CrossRef]
- Oliveira, J.A.C.; Kiala, G.; Siopa, F.; Bernard, A.; Gontard, G.; Oble, J.; Afonso, C.A.M.; Poli, G. Palladium-catalyzed allylic substitution between C-based nucleophiles and 6-azabicyclo[3.1.0]-hex-3-en-2-oxy derivatives: A new selectivity paradigm. Tetrahedron 2020, 76, 131182. [Google Scholar] [CrossRef]
- Ling, R.; Yoshida, M.; Mariano, P.S. Exploratory investigations probing a preparatively versatile, pyridinium salt photoelectrocyclization-solvolytic aziridine ring opening sequence. J. Org. Chem. 1996, 61, 4439–4449. [Google Scholar] [CrossRef]
- Acar, E.A.; Glarner, F.; Burger, U. Aminocyclopentitols from N-Alkylpyridinium Salts: A photochemical approach. Helv. Chim. Acta 1998, 81, 1095–1104. [Google Scholar] [CrossRef]
- Gleiter, C.H.; Jägle, C.; Gresser, U.; Mörike, K. Candesartan. Cardiovasc. Drug Rev. 2004, 22, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.G.S.; Hull, C.M.; Parker, J.E.; Garvey, E.P.; Hoekstra, W.J.; Moore, W.R.; Schotzinger, R.J.; Kelly, D.E.; Kellya, S.L. The clinical candidate VT-1161 is a highly potent inhibitor of candida albicans CYP51 but fails to bind the human enzyme. Antimicrob. Agents Chemother. 2014, 58, 7121–7127. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Fothergill, A.W.; Iqbal, N.; Bolden, C.B.; Grossman, N.T.; Garvey, E.P.; Brand, S.R.; Hoekstra, W.J.; Schotzinger, R.J.; Ottinger, E.; et al. The Investigational Fungal Cyp51 Inhibitor VT-1129 Demonstrates Potent In Vitro Activity against Cryptococcus neoformans and Cryptococcus gattii. Antimicrob. Agents Chemother. 2016, 60, 2528–2531. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, M.A.G.; Siopa, F.; Afonso, C.A.M. (1R,4S,5S)-5-((3-Hydroxypropyl)amino)-4-((1-methyl-1H-tetrazol-5-yl)thio)cyclopent-2-en-1-ol. Molbank 2021, 2021, M1199. https://doi.org/10.3390/M1199
Fortunato MAG, Siopa F, Afonso CAM. (1R,4S,5S)-5-((3-Hydroxypropyl)amino)-4-((1-methyl-1H-tetrazol-5-yl)thio)cyclopent-2-en-1-ol. Molbank. 2021; 2021(2):M1199. https://doi.org/10.3390/M1199
Chicago/Turabian StyleFortunato, Milene A. G., Filipa Siopa, and Carlos A. M. Afonso. 2021. "(1R,4S,5S)-5-((3-Hydroxypropyl)amino)-4-((1-methyl-1H-tetrazol-5-yl)thio)cyclopent-2-en-1-ol" Molbank 2021, no. 2: M1199. https://doi.org/10.3390/M1199
APA StyleFortunato, M. A. G., Siopa, F., & Afonso, C. A. M. (2021). (1R,4S,5S)-5-((3-Hydroxypropyl)amino)-4-((1-methyl-1H-tetrazol-5-yl)thio)cyclopent-2-en-1-ol. Molbank, 2021(2), M1199. https://doi.org/10.3390/M1199