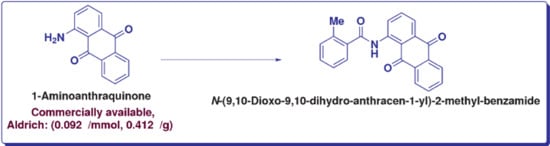
N-(9,10-Dioxo-9,10-dihydroanthracen-1-yl)-2-methylbenzamide
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
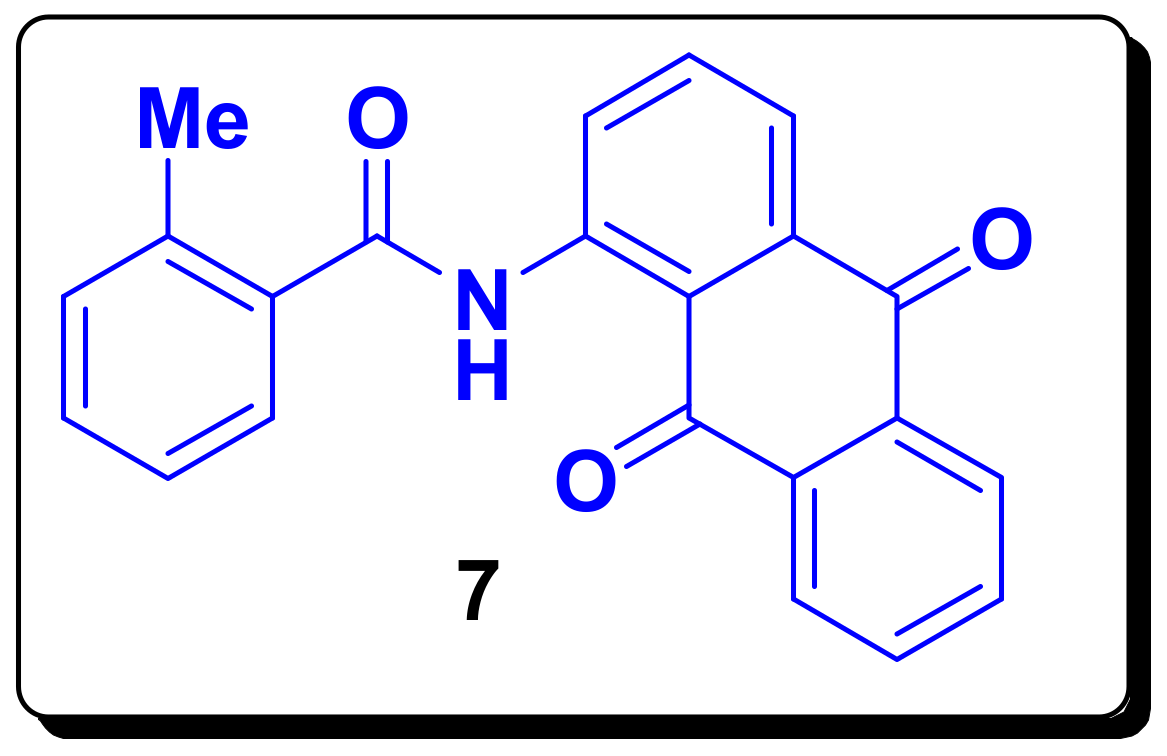
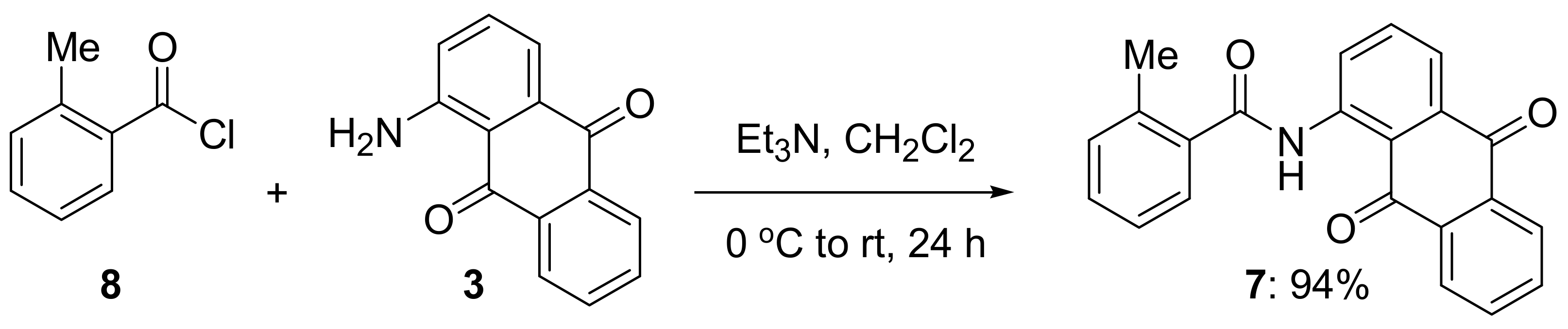
3.2. Synthesis of N-(9,10-Dioxo-9,10-dihydroanthracen-1-yl)-2-methylbenzamide (7)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shilov, A.E.; Shul’pin, G.B. Activation of C-H bonds by metal complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, A. Mechanisms of intramolecular activation of carbon-hydrogen bonds in transition-metal complexes. Chem. Rev. 1990, 90, 403–424. [Google Scholar] [CrossRef]
- Dick, A.R.; Sanford, M.S. Transition metal catalyzed oxidative functionalization of carbon-hydrogen bonds. Tetrahdedron 2006, 11, 2439–2463. [Google Scholar] [CrossRef]
- Li, J.; De Sarkar, S.; Ackermann, L. Meta- and para-selective C-H functionalization by C-H cctivation. Top. Organomet. Chem. 2016, 55, 217–257. [Google Scholar] [CrossRef]
- Sato, T.; Yoshida, T.; Al Mamari, H.H.; Ilies, L.; Nakamura, E. Manganese-catalyzed directed methylation of C(sp2)-H bonds at 25 °C with high catalytic turnover. Org. Lett. 2017, 19, 5458–5461. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnio, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. A Comprehensive review of directing groups applied in metal-catalyzed C-H functionalization chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef] [PubMed]
- Rouquet, G.; Chatani, N. Catalytic functionalization of C(sp2)-H and C(sp3)-H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [Google Scholar] [CrossRef] [PubMed]
- Omae, I. Intramolecular five-membered ring compounds and their applications. Coord. Chem. Rev. 2004, 248, 995–1023. [Google Scholar] [CrossRef]
- Al Mamari, H.H.; Diers, E.; Ackermann, L. Triazole-assisted ruthenium-catalyzed C-H arylation of aromatic amides. Chem. Eur. J. 2014, 20, 9739–9743. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Al Mamari, H.H.; Graczyk, K.; Diers, E.; Ackermann, L. Iron-catalyzed C(sp2)-H and C(sp3)-H arylation by triazole assistance. Angew. Chem. Int. Ed. 2014, 53, 3868–3871. [Google Scholar] [CrossRef] [PubMed]
- Al Mamari, H.H.; Al Awaimri, N.; Al Lawati, Y. N-Benzo[c][1,2,5]thiazol-4-yl-3—trifluoromethylbenzamide. Molbank 2019, 2019, M1075. [Google Scholar] [CrossRef]
- Al Mamari, H.H.; Al Lawati, Y. N-(2-Hydroxy-1,1-dimethylethyl)-3-methylbenzamide. Molbank 2020, 2020, M1099. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mamari, H.H.; Al Sheidi, A. N-(9,10-Dioxo-9,10-dihydroanthracen-1-yl)-2-methylbenzamide. Molbank 2020, 2020, M1175. https://doi.org/10.3390/M1175
Al Mamari HH, Al Sheidi A. N-(9,10-Dioxo-9,10-dihydroanthracen-1-yl)-2-methylbenzamide. Molbank. 2020; 2020(4):M1175. https://doi.org/10.3390/M1175
Chicago/Turabian StyleAl Mamari, Hamad H., and Ahmed Al Sheidi. 2020. "N-(9,10-Dioxo-9,10-dihydroanthracen-1-yl)-2-methylbenzamide" Molbank 2020, no. 4: M1175. https://doi.org/10.3390/M1175
APA StyleAl Mamari, H. H., & Al Sheidi, A. (2020). N-(9,10-Dioxo-9,10-dihydroanthracen-1-yl)-2-methylbenzamide. Molbank, 2020(4), M1175. https://doi.org/10.3390/M1175