Synthesis, Structure and Hirshfeld Surface Analysis of Phosphine–Imidazolium Salt
Abstract
:1. Introduction
2. Results
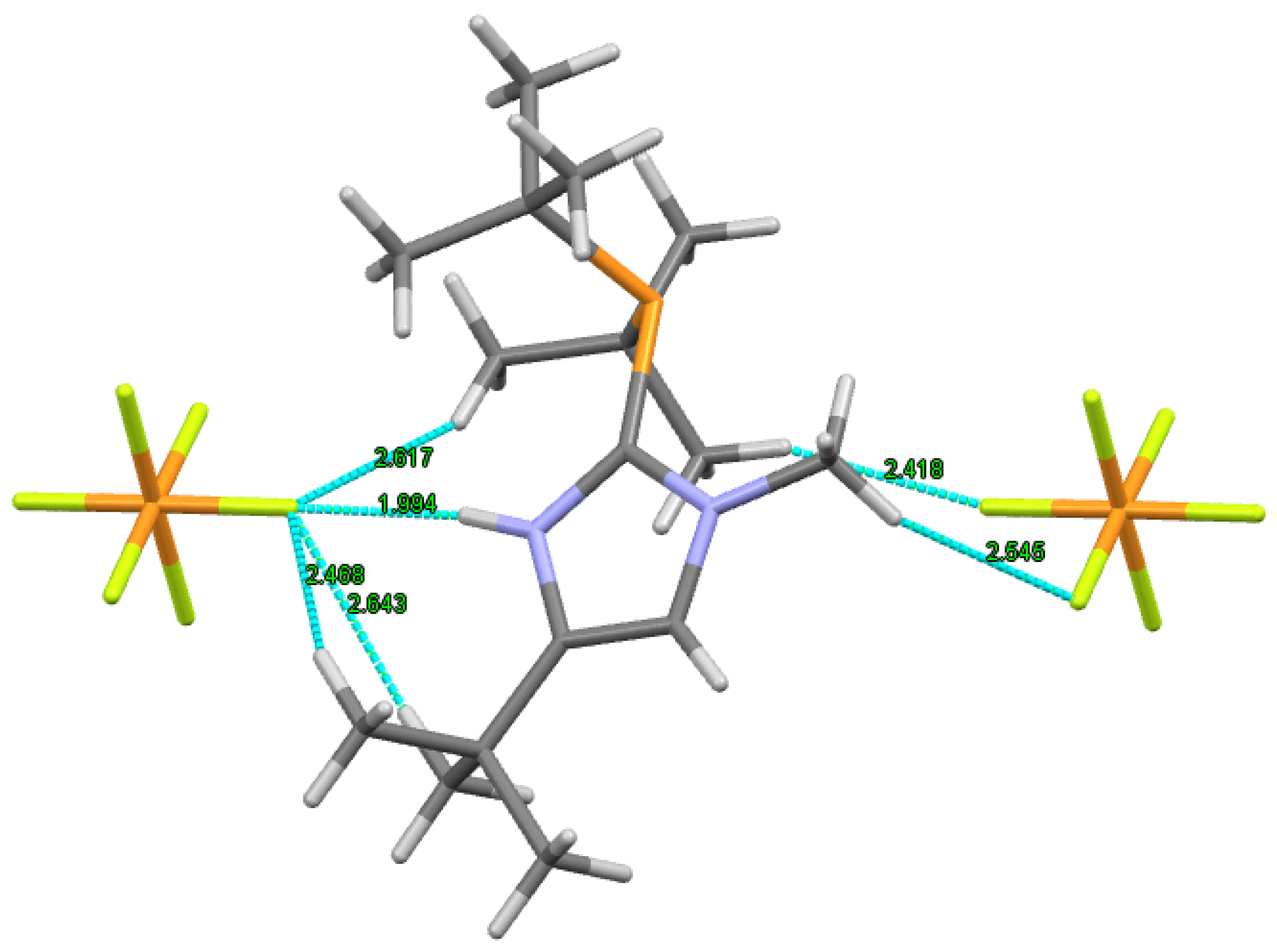
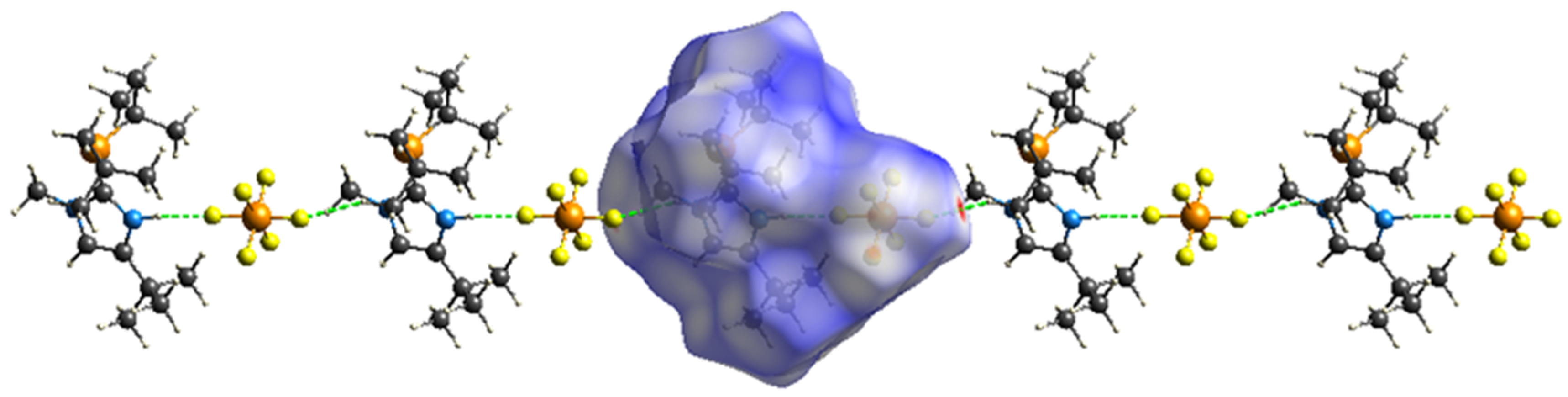
Hirschfeld Surface Analysis
3. Materials and Methods
Synthesis of Phosphine-Imidazolium Salt [tBu2PImH]PF6
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grotjahn, D.B.; Incarvito, C.D.; Rheingold, A.L. Combined effects of metal and ligand capable of accepting a proton or hydrogen bond catalyze anti-Markovnikov hydration of terminal alkynes. Angew. Chem. Int. Ed. 2001, 40, 3884–3887. [Google Scholar] [CrossRef]
- Grotjahn, D.B.; Larsen, C.R.; Gustafson, J.L.; Nair, R.; Sharma, A. Extensive isomerization of alkenes using a bifunctional catalyst: An alkene zipper. J. Am. Chem. Soc. 2007, 129, 9592–9593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdogan, G.; Grotjahn, D.B. Mild and selective deuteration and isomerization of alkenes by a bifunctional catalyst and deuterium oxide. J. Am. Chem. Soc. 2009, 131, 10354–10355. [Google Scholar] [CrossRef] [PubMed]
- Grotjahn, D.B.; Gong, Y.; Zakharov, L.; Golen, J.A.; Rheingold, A.L. Changes in coordination of sterically demanding hybrid imidazolylphosphine ligands on Pd(0) and Pd(II). J. Am. Chem. Soc. 2006, 128, 438–453. [Google Scholar] [PubMed]
- Smarun, A.V.; Shahreel, W.; Pramono, S.; Koo, S.Y.; Tan, L.Y.; Ganguly, R.; Vidović, D. Influence of increasing steric demand on isomerization of terminal alkenes catalysed by bifunctional ruthenium complexes. J. Organomet. Chem. 2017, 834, 1–9. [Google Scholar] [CrossRef]
- Smarun, A.V.; Petković, M.; Shchepinov, M.S.; Vidović, D. Site-specific deuteration of polyunsaturated alkenes. J. Org. Chem. 2017, 82, 13115–13120. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Cui, X.-Y.; Tan, S.M.; Jiang, H.; Ren, J.; Lee, N.; Lee, R.; Tan, C.-H. Guanidine–Copper Complex Catalyzed Allylic Borylation for the Enantioconvergent Synthesis of Tertiary Cyclic Allylboronates. Angew. Chem. Int. Ed. Engl. 2019, 58, 2382–2383. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
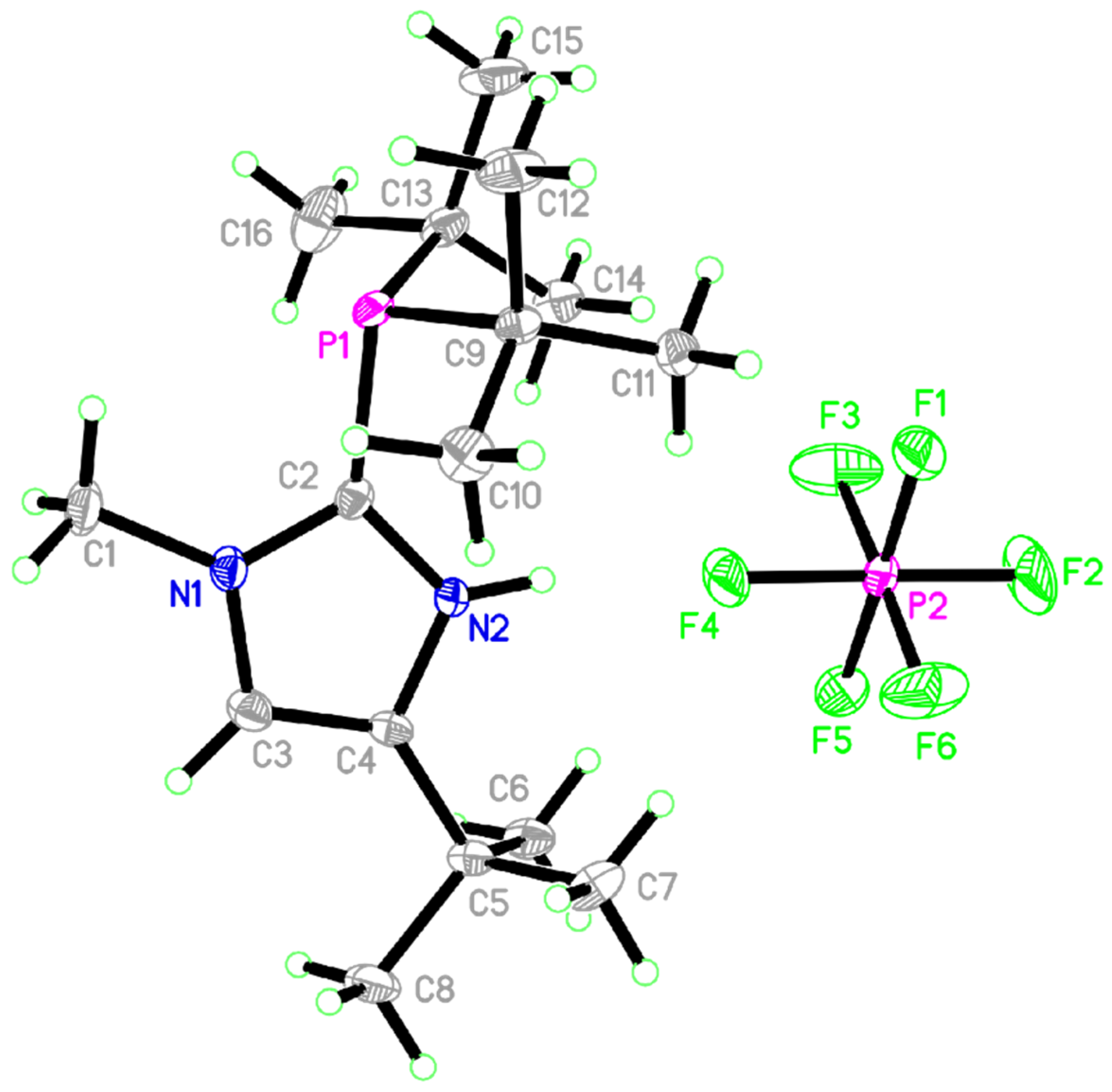

| Bond Lengths (Å) | Bond Angles (deg) | ||
|---|---|---|---|
| P1–C2 | 1.8395(2) | C2 -P1 -C9 | 105.05(1) |
| P1–C9 | 1.8858(2) | C2 -P1 -C13 | 130.96(1) |
| P1–C13 | 1.8807(2) | C9 -P1 -C13 | 123.96(1) |
| P2–F2 | 1.5829(1) | F2 -P2 -F5 | 117.97(1) |
| P2–F4 | 1.6102(1) | F3 -P2 -F4 | 107.63(1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smarun, A.V.; Jevtovic, V.; Ganguly, R. Synthesis, Structure and Hirshfeld Surface Analysis of Phosphine–Imidazolium Salt. Molbank 2020, 2020, M1141. https://doi.org/10.3390/M1141
Smarun AV, Jevtovic V, Ganguly R. Synthesis, Structure and Hirshfeld Surface Analysis of Phosphine–Imidazolium Salt. Molbank. 2020; 2020(2):M1141. https://doi.org/10.3390/M1141
Chicago/Turabian StyleSmarun, Alexey V., Violeta Jevtovic, and Rakesh Ganguly. 2020. "Synthesis, Structure and Hirshfeld Surface Analysis of Phosphine–Imidazolium Salt" Molbank 2020, no. 2: M1141. https://doi.org/10.3390/M1141
APA StyleSmarun, A. V., Jevtovic, V., & Ganguly, R. (2020). Synthesis, Structure and Hirshfeld Surface Analysis of Phosphine–Imidazolium Salt. Molbank, 2020(2), M1141. https://doi.org/10.3390/M1141






