Abstract
A new pyrrole-substituted terpyridine derivative that possesses an allene moiety was obtained as an “unexpected” sole product during an attempt to alkylate the N-atom of pyrrole with propargyl bromide in order to obtain an alkyne-functionalized terpyridine.
1. Introduction
Terpyridine ligands (terpy) and their complexes have been widely studied [1]. This can be easily explained by the huge number of terpyridine derivatives that can be obtained by varying the substitution pattern of the ligand as well as the nature of the complexed metal. In particular, terpyridines that contain a five membered heterocycle, such as furan [2] or thiophene [3], have attracted a lot of attention. In fact, they can be used as intermediates in the preparation of materials for solar cells [4] or nanoparticles [5], as biological probes [6], as ligands in catalysis [7], as antimicrobial agents [8], as electrochromic materials [9] or as chromophores [10], to name just a few applications. Although a little less studied, terpyridines that include a pyrrole ring have also been a subject of interest. For example, such terpyridines have been used for the preparation of cytotoxic molecules [11], for application in OLED (organic light emitting diodes) [12] or sensor devices [13] or for the preparation of catalytic materials [14]. Thus, the preparation of terpyridine derivatives that contain a functionalized pyrrole is of interest in the fields of both organic synthesis and material science. This article describes how pyrrole-containing terpyridine 1 was obtained as an unexpected product during attempts to prepare compound 2 (Figure 1), which features an alkyne chain for possible future functionalization [15].
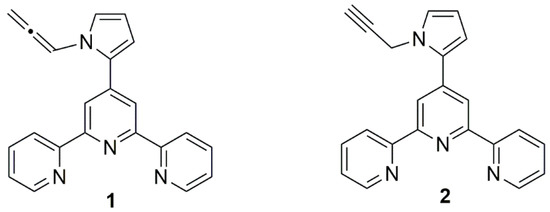
Figure 1.
Structures of terpyridine compounds (1) and (2).
2. Results and Discussion
The synthetic approach towards molecule 2 relies on the N-alkylation of the pyrrole moiety of 4′-(pyrrol-2-yl)-2,2′:6′,2″-terpyridine (3) with propargyl bromide (Figure 2), applying a protocol that has been described for the preparation of N-alkyl terpyridine pyrroles [16]. At the end of the reaction, a single product was noticed by TLC. This product was easily separated from the starting material by flash chromatography, but 1H NMR did not agree with the structure of 2.
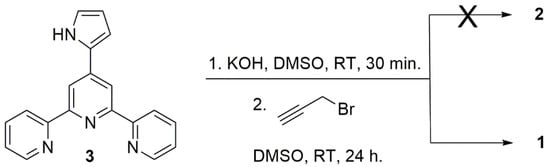
Figure 2.
Synthetic pathway that afforded compound 1.
Instead, structure 1 was coherent with 1H NMR. In particular, the spectrum exhibits the signals for the allenic protons. In fact, a triplet is observed at 7.14 ppm (J = 6.4 Hz). This signal accounts for the allenic proton e (Figure 3). In addition, a doublet is observed at 5.50 ppm (J = 6.4 Hz) for the allenic protons g. These multiplicities and chemical shifts are in accordance with those reported for other allene-functionalized pyrroles [17,18,19].
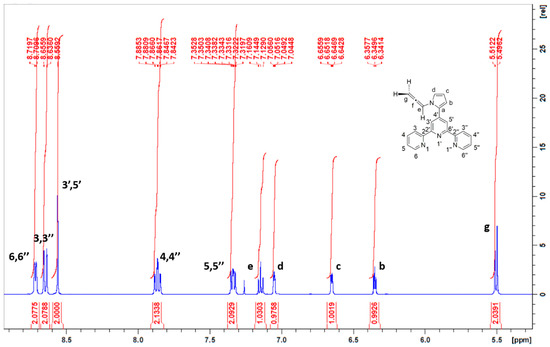
Figure 3.
1H NMR spectra of compound 1 (inset: structure and atom numbering of 1).
All other signals arising from the terpyridine and the pyrrole parts of the molecule are present. Additionally, the structure of 1 was further confirmed by Supplementary Materials 13C NMR, as well as by HR-MS. For instance, the 13C NMR spectrum features 15 signals due to the symmetry of the molecule, while mass spectra exhibit the molecular ion peak at 337.14466 (calc. for [C22H16N4 + H]+: 337.14477).
As pointed out in the above-mentioned literature, the formation of compound 1 is not so “unexpected”. Nevertheless, no trace of alkyne 2 was noticed, while N-propargylation of pyrrole and indole derivatives under similar reaction conditions are described in the literature [20,21]. Therefore, the obtention of 1 as the sole product is “unexpected”.
3. Materials and Methods
All reagents were purchased from commercial suppliers and used as received. The 4′-(Pyrrol-2-yl)-2,2′:6′,2″-terpyridine (3) was prepared according to the literature [16]. A volume of 85% Potassium hydroxide pellets (VWR Chemicals, France) was powdered using a mortar and a pestle. Anhydrous DMSO and 80% propargyl bromide solution in toluene were purchased from ACROS Organics (Geel, Belgium) and used as received. Flash chromatography was carried out on a Combiflash Rf+ Lumen (Teledyne ISCO, Lincoln, NE, USA) using a Redisep Rf neutral alumina column (Teledyne ISCO, Lincoln, NE, USA) with a hexane/ethyl acetate mixture (100:0 to 80:20 v:v) as eluent. The 1H and 13C NMR spectra were recorded on a Brucker AC 400 (Bruker, Wissembourg, France) at 400 and 100 MHz, respectively, using CDCl3 as a solvent. The melting point was recorded with a Stuart SMP 10 melting point apparatus (Bibby Sterilin, Stone, UK) and was uncorrected. HR-MS was recorded at Sayence SATT, Dijon, France.
4′-(N-(Propan-1,2-dienyl)pyrrol-2-yl)-2,2′:6′,2″-terpyridine (1):Into a round bottomed flask, the powdered potassium hydroxide (0.44 g; 6.7 mmol) and dimethylsulfoxide (35 mL) were successively placed. The resulting suspension was stirred at room temperature under argon for 30 minutes. Then, 4′-(pyrrol-2-yl)-2,2′:6′,2″-terpyridine (1.00 g; 3.35 mmol) was added and the red solution was stirred at room temperature under argon for 30 min. Finally, propargyl bromide (80% solution in toluene, 0.50 g; 3.36 mmol) was added and the reaction mixture was stirred at room temperature under argon for 24 h. The solution was then poured onto water (100 mL) and a small amount of brine was added to ensure proper decantation. The aqueous layer was extracted with dichloromethane (4 × 25 mL). The organic layers were combined, washed with brine (100 mL), dried over sodium sulfate and concentrated under vacuo. The crude was purified by flash chromatography. The title compound was obtained as a white solid (0.52–0.66 g) Mp = 128 °C. 1H NMR (CDCl3, 400 MHz), δ (ppm): 8.71 (d, 2H H6, 6’’, J = 4.0 Hz), 8.65 (d, 2H, H3, 3’’, J = 8.0 Hz), 8.56 (s, 2H, H3’, 5’), 7.86 (td, 2H, H4, 4’’, J = 7.7 Hz, J = 1.7 Hz), 7.34 (ddd, 2H, H5, 5’’, J = 7.4 Hz, J = 4.8 Hz, J = 1.0 Hz), 7.14 (t, 1H, He, J = 6.4 Hz), 7.05 (dd, 1H, Hd, J = 2.7 Hz, J = 1.8 Hz), 6.65 (dd, 1H, Hc, J = 3.6 Hz, J = 1.6 Hz), 6.35 (t, 1H, Hb, J = 3.2 Hz), 5.50 (d, 1H, Hg, J = 6.4 Hz). 13C NMR (CDCl3, 100 MHz), δ (ppm): 203.5, 156.2, 155.7, 149.2, 141.8, 136.8, 131.6, 123.8, 122.7, 121.3, 119.9, 112.4, 110.4, 98.7, 87.2. HR-MS: calc. for [C22H16N4 + H]+ 337.14477, found 337.14466.
4. Conclusions
A new pyrrole-containing terpyridine has been prepared and characterized. It features an allenic part that is linked via the N-atom of the pyrrole nucleus. Considering the fact that allenes are valuable intermediates in the preparation of polymer [22] or molecular materials [23], and the impressive metal-coordination properties of terpyridine ligands [1], this new compound could be useful for the fabrication of novel metal-containing functional materials.
Supplementary Materials
The following are available online, 1H, 13C, 1H-1H COSY and 1H-13C HSQC NMR, HR-MS spectra of terpyridine 1.
Author Contributions
J.H. conceived and carried out the experiments, analyzed data and prepared the manuscript. L.G. analyzed data and contributed to manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive specific funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Husson, J.; Knorr, M. Syntheses and applications of furanyl-functionalised 2,2′:6′,2″-terpyridines. Beilstein J. Org. Chem. 2012, 8, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Husson, J.; Knorr, M. 2,2′:6′,2″-Terpyridines Functionalized with Thienyl Substituents: Synthesis and Applications. J. Heterocyclic Chem. 2012, 49, 453–478. [Google Scholar] [CrossRef]
- Dehaudt, J.; Husson, J.; Guyard, L. A more efficient synthesis of 4,4′,4″-tricarboxy-2,2′:6′,2″-terpyridine. Green Chem. 2011, 13, 3337–3340. [Google Scholar] [CrossRef]
- Momeni, B.Z.; Doustkhahvajari, F. Heteroleptic complexes of silver(I) featuring 4′-hydroxy and 4′-(2-furyl)-2,2′:6′,2″-terpyridine: An easy route for synthesis of silver nanoparticles. Inorg. Chim. Acta. 2019, 487, 145–152. [Google Scholar] [CrossRef]
- Shen, Y.; Shao, T.; Fang, B.; Du, W.; Zhang, M.; Liu, J.; Liu, T.; Tian, X.; Zhang, Q.; Wang, A.; et al. Visualization of mitochondrial DNA in living cells with super-resolution microscopy using thiophene-based terpyridine Zn(II) complexes. Chem. Commun. 2018, 54, 11288–11291. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. 4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine: A New Ligand Obtained from a Biomass-Derived Aldehyde with Potential Application in Metal-Catalyzed Reactions. Molbank 2018, 2018, M1032. [Google Scholar] [CrossRef]
- Njogu, E.M.; Martincigh, B.S.; Omondi, B.; Nyamori, V.O. Synthesis, characterization, antimicrobial screening and DNA binding of novel silver(I)-thienylterpyridine and silver(I)-furylterpyridine. Appl. Organomet. Chem. 2018, 32, E4554. [Google Scholar] [CrossRef]
- Liang, Y.W.; Strohecker, D.; Lynch, V.; Holliday, B.J.; Jones, R.A. A Thiophene-Containing Conductive Metallopolymer Using an Fe(II) Bis(terpyridine) Core for Electrochromic Materials. ACS Appl. Mater. Interfaces 2016, 8, 34568–34580. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Besley, M.; Ciarrocchi, C.; Licchelli, M.; Raposo, M.M.M. Terpyridine derivatives functionalized with (hetero)aromatic groups and the corresponding Ru complexes: Synthesis and characterization as SHG chromophores. Dyes Pigment. 2018, 150, 49–58. [Google Scholar] [CrossRef]
- Czerwinska, K.; Machura, B.; Kula, S.; Krompiec, S.; Erfurt, K.; Roma-Rodrigues, C.; Fernandes, A.R.; Shul’pina, L.; Ikonnikov, N.S.; Shul’pin, G.B. Copper(II) complexes of functionalized 2,2′:6′,2″-terpyridines and 2,6-di(thiazol-2-yl)pyridine: Structure, spectroscopy, cytotoxicity and catalytic activity. Dalton Trans. 2017, 46, 9591–9604. [Google Scholar] [CrossRef]
- Klemens, T.; Switlicka-Olszewska, A.; Machura, B.; Grucela, M.; Schab-Balcerzak, E.; Smolarek, K.; Mackowski, S.; Szlapa, A.; Kula, S.; Krompiec, S.; et al. Rhenium(I) terpyridine complexes—Synthesis, photophysical properties and application in organic light emitting devices. Dalton Trans. 2016, 45, 1746–1762. [Google Scholar] [CrossRef] [PubMed]
- Naidji, B.; Husson, J.; Et Taouil, A.; Brunol, E.; Sanchez, J.-B.; Berger, F.; Rauch, J.-Y.; Guyard, L. Terpyridine-based metallopolymer thin films as active layer in ammonia sensor device. Synth. Met. 2016, 221, 214–219. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. A new and facile method for the functionalization of a Merrifield resin with terpyridines: Application as a heterogeneous catalyst for the synthesis of biaryls in environmentally friendly solvents. Green Process. Synth. 2016, 5, 331–336. [Google Scholar] [CrossRef]
- Busemann, A.; Araman, C.; Flaspohler, I.; Pratesi, A.; Zhou, X.-Q.; van Rixel, V.H.S.; Siegler, M.A.; Messori, L.; van Kasteren, S.I.; Bonnet, S. Alkyne Functionalization of a Photoactivated Ruthenium Polypyridyl Complex for Click-Enabled Serum Albumin Interaction Studies. Inorg. Chem. 2020, 59, 7710–7720. [Google Scholar] [CrossRef] [PubMed]
- Husson, J.; Guyard, L. Synthesis of new 4′-(N-alkylpyrrol-2-yl)-2,2′:6′,2″-terpyridines via N-alkylation of a pyrrole moiety. Heterocycl. Commun. 2015, 21, 199–202. [Google Scholar] [CrossRef]
- Tarasova, O.A.; Brandsma, L.; Trofimov, B.A. Facile One-Pot Syntheses of 1-Allenylpyrroles. Synthesis 1993, 571–572. [Google Scholar] [CrossRef]
- Tarasova, O.A.; Taherirastgar, F.; Verkruijsse, H.D.; Mal’kina, A.G.; Brandsma, L.; Trofimov, B.A. Metallation and functionalization of 1-allenylpyrrole. Recl. Trav. Chim. Pays Bas 1996, 115, 145–147. [Google Scholar] [CrossRef]
- Duan, G.-J.; Ling, J.-B.; Wang, W.-P.; Luo, Y.-C.; Xu, P.-F. Organocatalytic formal [2+2] cycloaddition initiated by vinylogous Friedel-Crafts alkylation: Enantioselective synthesis of substituted cyclobutene derivatives. Chem. Commun. 2013, 49, 4625–4627. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.; Xu, M.; Zheng, Z.; Zhang, R.; Li, Y. Selective synthesis of pyrrolo[1,2-a]azepines or 4,6-dicarbonyl indoles via tandem reactions of alkynone with pyrrole derivatives. Org. Biomol. Chem. 2017, 15, 6328–6332. [Google Scholar] [CrossRef]
- Turnu, F.; Luridiana, A.; Cocco, A.; Porcu, S.; Frongia, A.; Sarais, G.; Secci, F. Catalytic Tandem Friedel-Crafts Alkylation/C4-C3 Ring-Contraction Reaction: An Efficient Route for the Synthesis of Indolyl Cyclopropanecarbaldehydes and Ketones. Org. Lett. 2019, 21, 7329–7332. [Google Scholar] [CrossRef]
- Endo, T.; Tomita, I. Novel Polymerization Methods for Allene Derivatives. Prog. Polym. Sci. 1997, 22, 565–600. [Google Scholar] [CrossRef]
- Rivera-Fuentes, P.; Diederich, F. Allenes in Molecular Materials. Angew. Chem. Int. Ed. 2012, 51, 2818–2828. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).