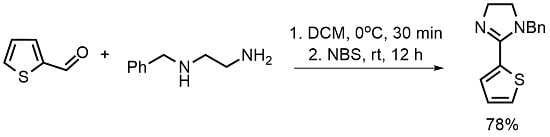
1-Benzyl-2-(thien-2-yl)-4,5-dihydro-1H-imidazole
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General
4.2. 1-Benzyl-2-(thien-2-yl)-4,5-dihydro-1H-imidazole (4)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Soni, J.; Sethiya, A.; Sahiba, N.; Agarwal, D.K.; Agarwal, S. Contemporary Progress in the Synthetic Strategies of Imidazole and its Biological Activities. Curr. Org. Syn. 2019, 16, 1078–1104. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, S.H.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Palta, K.; Kumar, M.; Bhargava, M.; Dahiya, L. Therapeutic potential of oxazole scaffold: A patent review (2006–2017). Expert Opin. Ther. Pat. 2018, 28, 783–812. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Silva, A.; McQuade, J.; Szostak, M. Recent Advances in the Synthesis and Reactivity of Isothiazoles. Adv. Synth. Catal. 2019, 361, 3050–3067. [Google Scholar] [CrossRef]
- Maftei, C.V.; Fodor, E.; Jones, P.G.; Daniliuc, C.G.; Franz, M.H.; Kelter, G.; Fiebig, H.H.; Tamm, M.; Neda, I. Novel 1,2,4-oxadiazoles and trifluoromethylpyridines related to natural products: Synthesis, structural analysis and investigation of their antitumor activity. Tetrahedron 2016, 72, 1185–1199. [Google Scholar] [CrossRef]
- Shaik, A.; Bhandare, R.R.; Palleapati, K.; Nissankararao, S.; Kancharlapalli, V.; Shaik, S. Antimicrobial, antioxidant, and anticancer activities of some novel isoxazole ring containing chalcone and dihydropyrazole derivatives. Molecules 2020, 25, 1047. [Google Scholar] [CrossRef]
- Kotian, S.Y.; Mohan, C.D.; Merlo, A.A.; Rangappa, S.; Nayak, S.C.; Rai, K.M.L.; Rangappa, K.S. Small molecule based five-membered heterocycles: A view of liquid crystalline properties beyond the biological applications. J. Mol. Liq. 2020, 297, 111686. [Google Scholar] [CrossRef]
- Kadyrov, A.; Neda, I.; Kaukorat, T.; Sonnenburg, R.; Fischer, A.; Jones, P.G.; Schmutzler, R. New Phospholene and Phosphepine Derivatives from λ3-Phosphorus Compounds and Hexafluoroacetone or Perfluorinated α-Diketones. Chem. Ber. 1996, 129, 725–732. [Google Scholar] [CrossRef]
- Regunathan, S.; Reis, D.J. Imidazoline receptors and their endogenous ligands. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 511–544. [Google Scholar] [CrossRef]
- Khan, Z.P.; Ferguson, C.N.; Jones, R.M. Alpha-2 and imidazoline receptor agonists therapeutic role. Anaesthesia. 1999, 54, 146–165. [Google Scholar] [CrossRef]
- Li, J.X. Imidazoline I2 receptors: An update. Pharmacol. Ther. 2017, 178, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.D.; Tochowicz, A.; Tang, Y.; Cameron, M.D.; McCall, L.I.; Hirata, K.; Siqueira-Neto, J.L.; Reed, S.L.; McKerrow, J.H.; Roush, W.R. Synthesis and Evaluation of Oxyguanidine Analogues of the Cysteine Protease Inhibitor WRR-483 against Cruzain. ACS Med. Chem. Lett. 2016, 7, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Krasavin, M. Biologically active compounds based on the privileged 2-imidazoline scaffold: The world beyond adrenergic/imidazoline receptor modulators. Eur. J. Med. Chem. 2015, 97, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, P.; Hudson, A.; García-Sevilla, J.A.; Li, J.X. Imidazoline receptor system: The past, the present, and the future. Pharmacol. Rev. 2020, 72, 50–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Peters, R. A highly strained planar-chiral platinacycle for catalytic activation of internal olefins in the Friedel-crafts alkylation of indoles. Angew. Chem. Int. Ed. 2009, 48, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, K.; Nakamura, S.; Shibata, N. Enantioselective aza-Morita-Baylis-Hillman reactions of acrylonitrile catalyzed by palladium(II) pincer complexes having C 2-symmetric chiral bis(imidazoline) ligands. Angew. Chem. Int. Ed. 2012, 51, 10337–10341. [Google Scholar] [CrossRef]
- Awata, A.; Arai, T. PyBidine/copper catalyst: Asymmetric exo’-selective [3+2] cycloaddition using imino ester and electrophilic indole. Angew. Chem. Int. Ed. 2014, 53, 10462–10465. [Google Scholar] [CrossRef]
- Kondo, M.; Nishi, T.; Hatanaka, T.; Funahashi, Y.; Nakamura, S. Catalytic Enantioselective Reaction of α-Aminoacetonitriles Using Chiral Bis(imidazoline) Palladium Catalysts. Angew. Chem. Int. Ed. 2015, 54, 8198–8202. [Google Scholar] [CrossRef]
- Dar’in, D.; Krasavin, M. The Chan-Evans-Lam N-Arylation of 2-Imidazolines. J. Org. Chem. 2016, 81, 12514–12519. [Google Scholar] [CrossRef]
- Fujioka, H.; Murai, K.; Ohba, Y.; Hirose, H.; Kita, Y. Intramolecular bromo-amination of 1,4-cyclohexadiene aminal: One-pot discrimination of two olefins and concise asymmetric synthesis of (−)-γ-lycorane. Chem. Commun. 2006, 832–834. [Google Scholar] [CrossRef]
- Lathrop, S.P.; Pompeo, M.; Chang, W.T.T.; Movassaghi, M. Convergent and Biomimetic Enantioselective Total Synthesis of (−)-Communesin F. J. Am. Chem. Soc. 2016, 138, 7763–7769. [Google Scholar] [CrossRef] [PubMed]
- Winkel, A.; Wilhelm, R. New chiral ionic liquids based on imidazolinium salts. Tetrahedron Asymmetry 2009, 20, 2344–2350. [Google Scholar] [CrossRef]
- Ishihara, M.; Togo, H. Facile preparation of 2-imidazolines from aldehydes with tert-butyl hypochlorite. Synthesis (Stuttg) 2007, 1939–1942. [Google Scholar] [CrossRef]
- Bai, G.Y.; Xu, K.; Chen, G.F.; Yang, Y.H.; Li, T.Y. A facile and efficient synthesis of 2-imidazolines from aldehydes using hydrogen peroxide and substoichiometric sodium iodide. Synthesis (Stuttg) 2011, 1599–1603. [Google Scholar] [CrossRef]
- Busacca, C.A.; Bartholomeyzik, T.; Cheekoori, S.; Grinberg, N.; Lee, H.; Ma, S.; Saha, A.; Shen, S.; Senanayake, C.H. On the Racemization of Chiral Imidazolines. J. Org. Chem. 2008, 73, 9756–9761. [Google Scholar] [CrossRef]
- Chen, J.Q.; Yu, W.L.; Wei, Y.L.; Li, T.H.; Xu, P.F. Photoredox-Induced Functionalization of Alkenes for the Synthesis of Substituted Imidazolines and Oxazolidines. J. Org. Chem. 2017, 82, 243–249. [Google Scholar] [CrossRef]
- Aksenov, A.V.; Aksenov, N.A.; Arutiunov, N.A.; Malyuga, V.V.; Ovcharov, S.N.; Rubin, M. Electrophilically activated nitroalkanes in reaction with aliphatic diamines en route to imidazolines. RSC Adv. 2019, 9, 39458–39465. [Google Scholar] [CrossRef]
- Fujioka, H.; Murai, K.; Kubo, O.; Ohba, Y.; Kita, Y. One-pot synthesis of imidazolines from aldehydes: Detailed study about solvents and substrates. Tetrahedron 2007, 63, 638–643. [Google Scholar] [CrossRef]
- Golantsov, N.E.; Festa, A.A.; Karchava, A.V.; Yurovskaya, M.A. Marine indole alkaloids containing an 1-(indol-3-yl)ethane-1,2-diamine fragment (Review). Chem. Heterocycl. Compd. 2013, 49, 203–225. [Google Scholar] [CrossRef]
- Golantsov, N.E.; Festa, A.A.; Varlamov, A.V.; Voskressensky, L.G. Revision of the Structure and Total Synthesis of Topsentin C. Synthesis (Stuttg) 2017, 49, 2562–2574. [Google Scholar] [CrossRef]
- Golantsov, N.E.; Festa, A.; Golubenkova, A.S.; Nguyen, K.M.; Yakovenko, E.A.; Varlamov, A.V.; Voskressensky, L.G. Total synthesis of hamacanthin B class marine bisindole alkaloids. Chem. Heterocycl. Compd. 2020, 56, 331–338. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubenkova, A.S.; Golantsov, N.E.; Festa, A.A.; Voskressensky, L.G. 1-Benzyl-2-(thien-2-yl)-4,5-dihydro-1H-imidazole. Molbank 2020, 2020, M1137. https://doi.org/10.3390/M1137
Golubenkova AS, Golantsov NE, Festa AA, Voskressensky LG. 1-Benzyl-2-(thien-2-yl)-4,5-dihydro-1H-imidazole. Molbank. 2020; 2020(2):M1137. https://doi.org/10.3390/M1137
Chicago/Turabian StyleGolubenkova, Alexandra S., Nikita E. Golantsov, Alexey A. Festa, and Leonid G. Voskressensky. 2020. "1-Benzyl-2-(thien-2-yl)-4,5-dihydro-1H-imidazole" Molbank 2020, no. 2: M1137. https://doi.org/10.3390/M1137
APA StyleGolubenkova, A. S., Golantsov, N. E., Festa, A. A., & Voskressensky, L. G. (2020). 1-Benzyl-2-(thien-2-yl)-4,5-dihydro-1H-imidazole. Molbank, 2020(2), M1137. https://doi.org/10.3390/M1137