2,9-Dimethyl-11-(3-pentadecylphenoxy)dibenzo[c,f][1,2,5]dithiaphosphepine 11-oxide
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
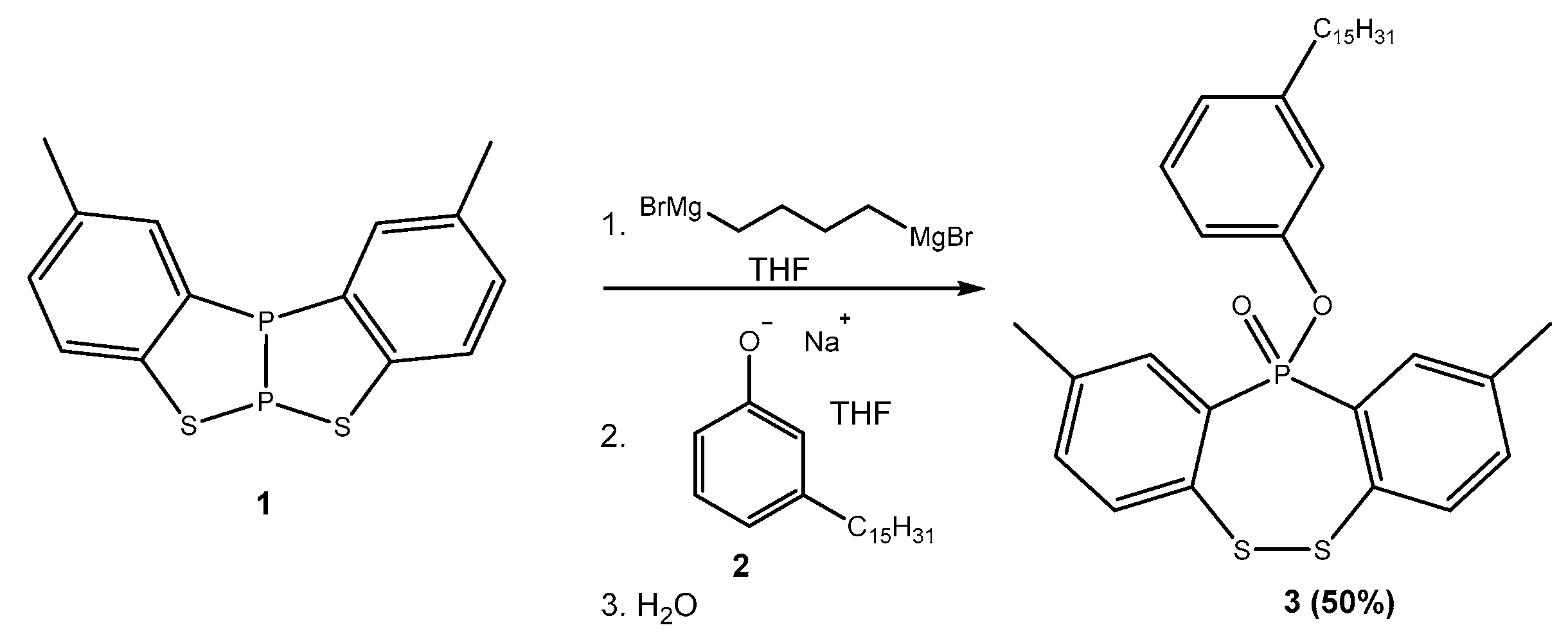
Synthesis of 2,9-Dimethyl-11-(3-pentadecylphenoxy)dibenzo[c,f][1,2,5]dithiaphosphepine 11-Oxide (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noyori, R. Asymmetric Catalysis in Organic Synthesis; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Burk, M.J.; Gross, M.F.; Martinez, J.P. Asymmetric catalytic synthesis of beta-branched amino acids via highly enantioselective hydrogenation of alpha-enamides. J. Am. Chem. Soc. 1995, 117, 9375–9376. [Google Scholar] [CrossRef]
- Jiang, Q.; Xiao, D.; Zhang, Z.; Cao, P.; Zang, X. Highly enantioselective hydrogenation of cyclic enol acetates catalyzed by a Rh–PennPhos complex. Angew. Chem. Int. Ed. 1999, 38, 516–518. [Google Scholar] [CrossRef]
- Baccolini, G.; Beghelli, M.; Boga, C. An improved synthesis of fused 1,2,3-benzothiadiphospholes and a proposed reaction pathway. Heteroatom Chem. 1997, 8, 551–556. [Google Scholar] [CrossRef]
- Baccolini, G.; Boga, C.; Mazzacurati, M.; Monari, M. Transformations of benzothiadiphosphole system: General one-pot synthesis of 1,2,5-dithiaphosphepines and their precursor phosphanethiols. Heteroatom Chem. 2005, 16, 339–345. [Google Scholar] [CrossRef]
- Baccolini, G.; Boga, C.; Mazzacurati, M.; Sangirardi, F. High atom-economical one-pot synthesis of secondary phosphines and their borane complexes using recycling phosphorus donor reagent. Org. Lett. 2006, 8, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Baccolini, G.; Micheletti, G.; Boga, C. The role played by phosphorus hexacoordination in driving the stereochemical outcome of a phosphination reaction. J. Org. Chem. 2009, 74, 6812–6818. [Google Scholar] [CrossRef] [PubMed]
- Boga, C.; Micheletti, G.; Delpivo, C.; Mazzacurati, M. A simple route to new cyclic (chloroalkyl)phosphane-, diphosphane-, and aminophosphane derivatives. Heteroatom Chem. 2013, 24, 392–397. [Google Scholar] [CrossRef]
- Baccolini, G.; Boga, C.; Mazzacurati, M.; Micheletti, G. The first flights of a molecular shuttle transporting elements: Easy one-pot formation of organic cyclic arsanes, stibanes and bismutanes. Chem. Eur. J. 2009, 15, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Baccolini, G.; Boga, C.; Guizzardi, G.; Ponzano, S. Simple and general synthesis of new 11H-11λ5-dibenzo[c, f][1,2,5]dithiaphosphepine derivatives. Tetrahedron Lett. 2002, 43, 9299–9302. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheletti, G.; Mazzacurati, M.; Telese, D.; Boga, C. 2,9-Dimethyl-11-(3-pentadecylphenoxy)dibenzo[c,f][1,2,5]dithiaphosphepine 11-oxide. Molbank 2020, 2020, M1109. https://doi.org/10.3390/M1109
Micheletti G, Mazzacurati M, Telese D, Boga C. 2,9-Dimethyl-11-(3-pentadecylphenoxy)dibenzo[c,f][1,2,5]dithiaphosphepine 11-oxide. Molbank. 2020; 2020(1):M1109. https://doi.org/10.3390/M1109
Chicago/Turabian StyleMicheletti, Gabriele, Marzia Mazzacurati, Dario Telese, and Carla Boga. 2020. "2,9-Dimethyl-11-(3-pentadecylphenoxy)dibenzo[c,f][1,2,5]dithiaphosphepine 11-oxide" Molbank 2020, no. 1: M1109. https://doi.org/10.3390/M1109
APA StyleMicheletti, G., Mazzacurati, M., Telese, D., & Boga, C. (2020). 2,9-Dimethyl-11-(3-pentadecylphenoxy)dibenzo[c,f][1,2,5]dithiaphosphepine 11-oxide. Molbank, 2020(1), M1109. https://doi.org/10.3390/M1109





