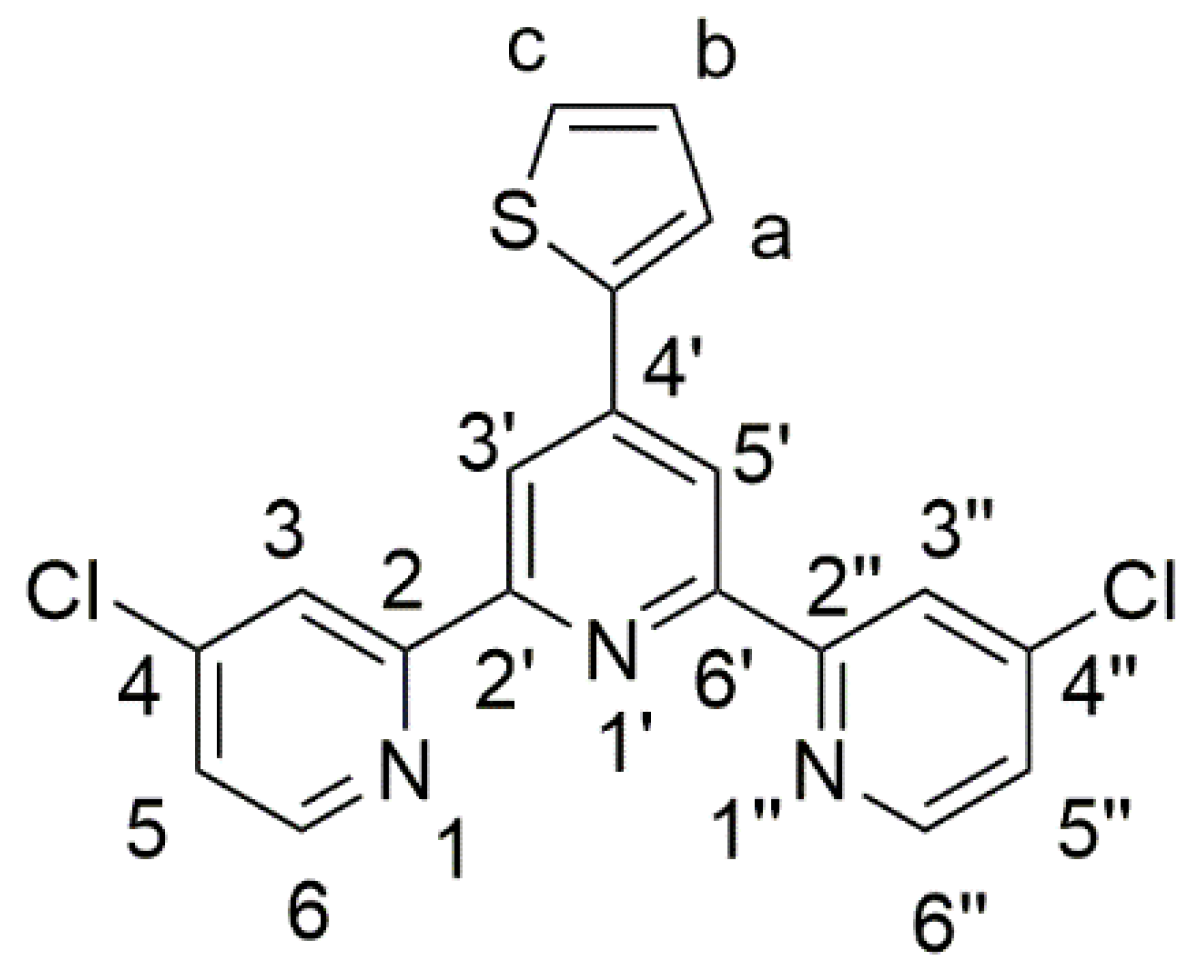
4,4″-Dichloro-4′-(2-thienyl)-2,2′:6′,2″-terpyridine
Abstract
1. Introduction
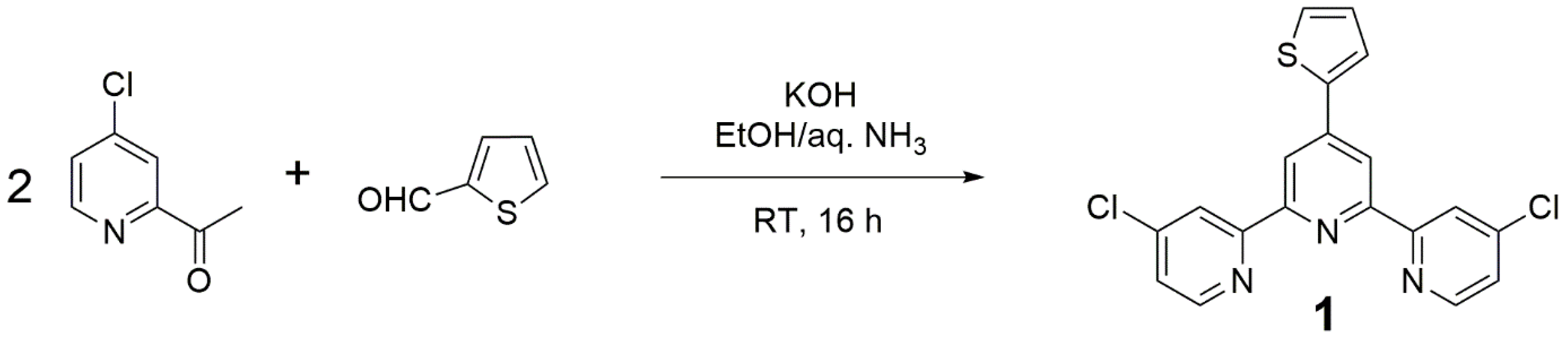
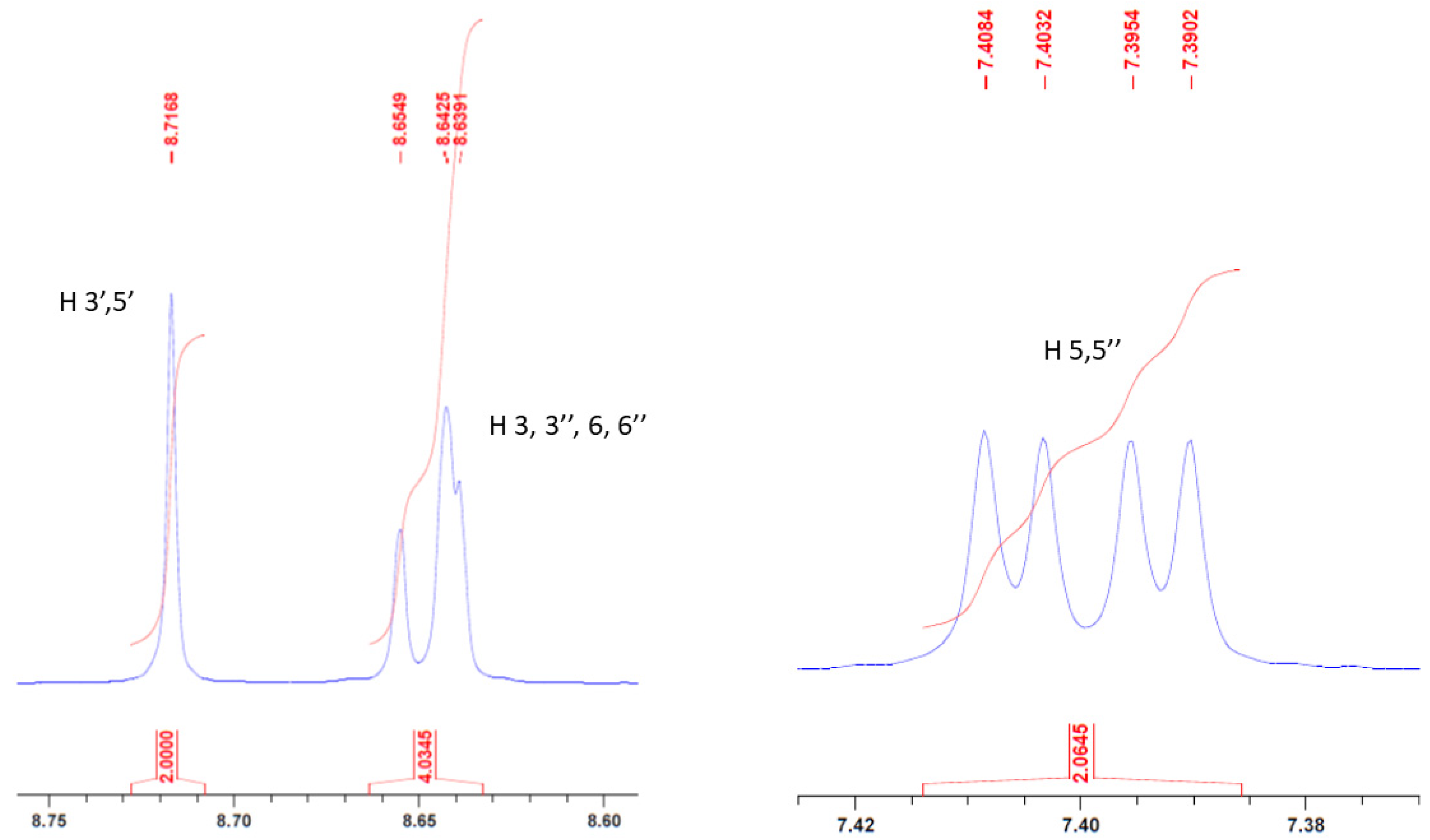
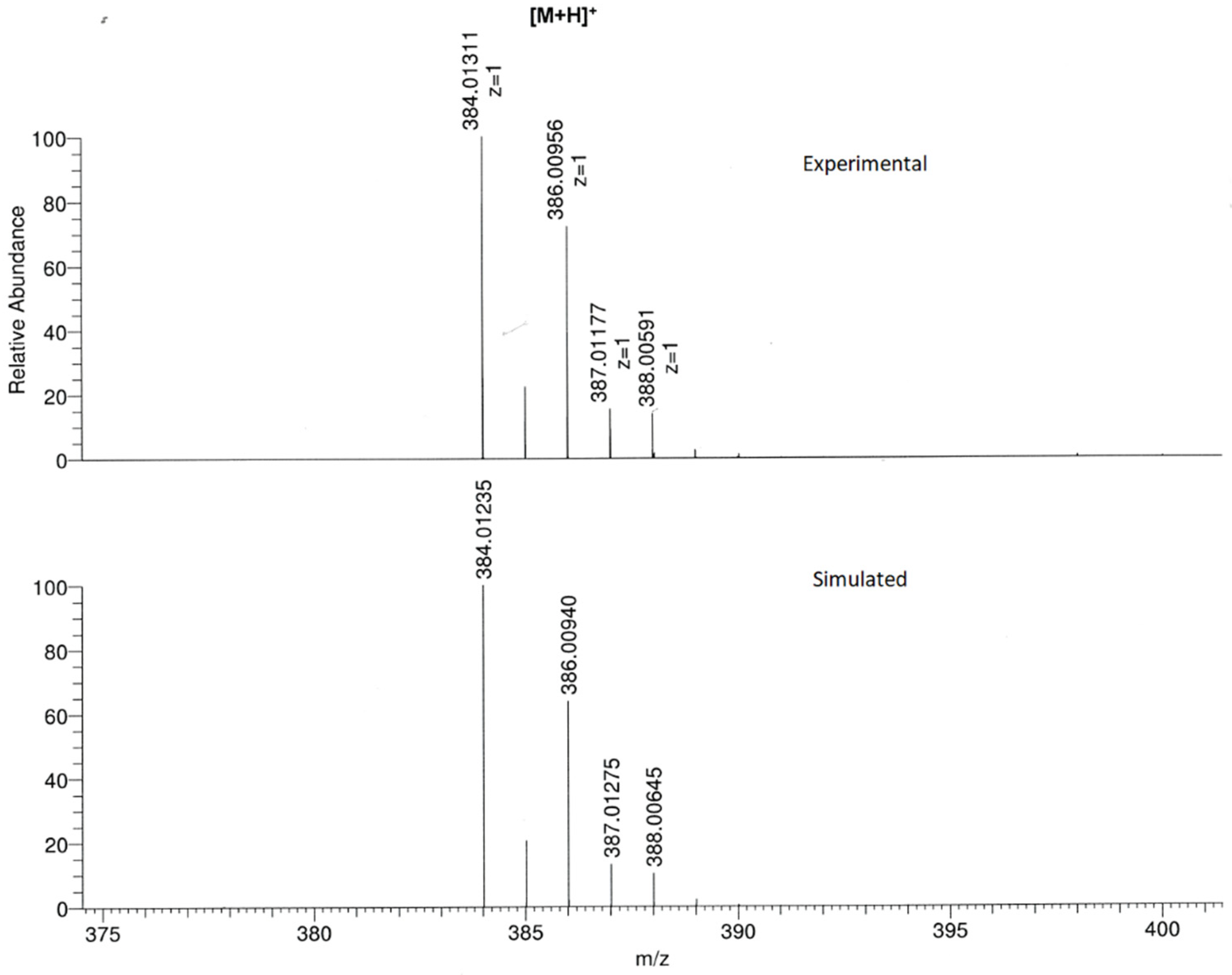
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of 4-Chloro-2-acetylpyridine
3.2. Preparation of 4,4″-Dichloro-4′-(2-thienyl)-2,2′:6′,2″-terpyridine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Saccone, D.; Magistris, C.; Barbero, N.; Quagliotto, P.; Barolo, C.; Viscardi, G. Terpyridine and Quaterpyridine Complexes as sensitizers for Photovoltaic Applications. Materials 2016, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Newkome, G.R.; Schubert, U.S. Catalytic Applications of Terpyridines and their Transition Metal Complexes. ChemCatChem 2011, 3, 1384–1406. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Nyamori, V.O.; Jonnalagadda, S.B.; Martincigh, B.S. Removal of Cd2+ and Hg2+ from aqueous solutions by adsorption onto nitrogen-functionalized carbon nanotubes. Desalin. Water Treat. 2018, 108, 253–267. [Google Scholar] [CrossRef]
- Allan, J.T.S.; Quaranta, S.; Ebralidze, I.I.; Egan, J.G.; Poisson, J.; Laschuk, N.O.; Gaspari, F.; Easton, E.B.; Zenkina, O.V. Terpyridine-Based Monolayer Electrochromic Materials. ACS Appl. Mater. Interfaces 2017, 9, 40438–40445. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.S.M.; Besley, M.; Ciarrocchi, C.; Licchelli, M.; Raposo, M.M.M. Terpyridine derivatives functionalized with (hetero)aromatic groups and the corresponding Ru complexes: Synthesis and characterization as SHG chromophores. Dyes Pigment. 2018, 150, 49–58. [Google Scholar] [CrossRef]
- Husson, J.; Knorr, M. 2,2′:6′,2″-Terpyridines Functionalized with Thienyl Substituents: Synthesis and Applications. J. Heterocyclic Chem. 2012, 49, 453–478. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Wang, Z.-F.; Wang, S.-L.; Luo, D.-M.; Zou, B.-Q.; Yao, P.-F.; Tan, M.-X.; Liang, H. In vitro and in vivo antitumor activities of three novel binuclear platinum(II) complexes with 4′-substituted-2,2′:6′,2″-terpyridine ligands. Eur. J. Med. Chem. 2019, 170, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shao, T.; Fang, B.; Du, W.; Zhang, M.; Liu, J.; Liu, T.; Tian, X.; Zhang, Q.; Wang, A.; et al. Visualization of mitochondrial DNA in living cells with super-resolution microscopy using thiophene-based terpyridine Zn(II) complexes. Chem. Commun. 2018, 54, 11288–11291. [Google Scholar] [CrossRef] [PubMed]
- Savan, E.K.; Koytepe, S.; Pasahan, A.; Erdogdu, G.; Seckin, T. Amperometric Simultaneous Measurement of Copper and Cobalt Ions with Polythiophene Incorporating Pendant Terpyridine Groups. Polym.-Plast. Technol. Eng. 2014, 53, 1817–1824. [Google Scholar] [CrossRef]
- Liang, Y.W.; Strohecker, D.; Lynch, V.; Holliday, B.J.; Jones, R.A. A Thiophene-Containing Conductive Metallopolymer Using an Fe(II) Bis(terpyridine) Core for Electrochromic Materials. ACS Appl. Mater. Interfaces 2016, 8, 34568–34580. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. 4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine: A new ligand obtained from a biomass-derived aldehyde with potential application in metal-catalyzed reactions. Molbank 2018, 2018, M1032. [Google Scholar] [CrossRef]
- Heller, M.; Schubert, U.S. Syntheses of functionalized 2,2′:6′,2″-terpyridines. Eur. J. Org. Chem. 2003, 6, 947–961. [Google Scholar] [CrossRef]
- Fallahpour, R.A. Synthesis of 4′-substituted-2,2′:6′,2″-terpyridines. Synthesis 2003, 2, 155–184. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The synthesis of 2,2′:6′,2″-terpyridine ligands- versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-Hindered 4′-Aryl-2,2′:6′,2″-Terpyridines. Synlett 2005, 8, 1251–1254. [Google Scholar] [CrossRef]
- Rocco, D.; Housecroft, C.E.; Constable, E.C. Synthesis of Terpyridines: Simple Reactions-What Could Possibly Go Wrong? Molecules 2019, 24, 1799. [Google Scholar] [CrossRef] [PubMed]
- Busto, E.; Gotor-Fernandez, V.; Gotor, V. Biocatalytic preparation of optically active 4-(N,N-dimethylamino)pyridines for application in chemical asymmetric catalysis. Tetrahedron-Asymmetry 2006, 17, 1007–1016. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. Trac-Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Harzmann, G.D.; Neuburger, M.; Mayor, M. 4,4″-Disubstituted Terpyridines and Their Homoleptic FeII Complexes. Eur. J. Inorg. Chem. 2013, 19, 3334–3347. [Google Scholar] [CrossRef]
- Love, B.E.; Jones, E.G. The Use of Salicylaldehyde Phenylhydrazone as an Indicator for the Titration of Organometallic Reagents. J. Org. Chem. 1999, 64, 3755–3756. [Google Scholar] [CrossRef]
- Lowe, G.; Droz, A.S.; Vilaivan, T.; Weaver, G.W.; Tweedale, L.; Pratt, J.M.; Rock, P.; Yardley, V.; Croft, S.L. Cytotoxicity of (2,2′:6′,2″-Terpyridine)platinum(II) Complexes to Leishmania donovani, Trypanosoma cruzi, and Trypanosoma brucei. J. Med. Chem. 1999, 42, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Bode, S.; Enke, M.; Bose, R.K.; Schacher, F.H.; Garcia, S.J.; van der Zwaag, S.; Hager, M.D.; Schubert, U.S. Correlation between scratch healing and rheological behaviour for terpyridine complex based metallopolymers. J. Mater. Chem. A 2015, 3, 22145–22153. [Google Scholar] [CrossRef]
- Mutai, T.; Cheon, J.-D.; Arita, S.; Araki, K. Phenyl-substituted 2,2′:6′,2″-terpyridine as a new series of fluorescent compounds-their photophysical properties and fluorescence tuning. J. Chem. Soc. Perkin Trans. 2 2001, 1045–1050. [Google Scholar] [CrossRef]
- Brandl, T.; Hoffmann, V.; Pannwitz, A.; Haussinger, D.; Neuburger, M.; Fuhr, O.; Bernhard, S.; Wenger, O.S.; Mayor, M. Chiral macrocyclic terpyridine complexes. Chem. Sci. 2018, 9, 3837–3843. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Bark, B.; Szymczak, N.K. Simple Ligand Modifications with Pendent OH Groups Dramatically Impact the Activity and Selectivity of Ruthenium Catalysts for Transfer Hydrogenation: The Importance of Alkali Metals. ACS Catal. 2016, 6, 1981–1990. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husson, J.; Guyard, L. 4,4″-Dichloro-4′-(2-thienyl)-2,2′:6′,2″-terpyridine. Molbank 2019, 2019, M1071. https://doi.org/10.3390/M1071
Husson J, Guyard L. 4,4″-Dichloro-4′-(2-thienyl)-2,2′:6′,2″-terpyridine. Molbank. 2019; 2019(3):M1071. https://doi.org/10.3390/M1071
Chicago/Turabian StyleHusson, Jérôme, and Laurent Guyard. 2019. "4,4″-Dichloro-4′-(2-thienyl)-2,2′:6′,2″-terpyridine" Molbank 2019, no. 3: M1071. https://doi.org/10.3390/M1071
APA StyleHusson, J., & Guyard, L. (2019). 4,4″-Dichloro-4′-(2-thienyl)-2,2′:6′,2″-terpyridine. Molbank, 2019(3), M1071. https://doi.org/10.3390/M1071





