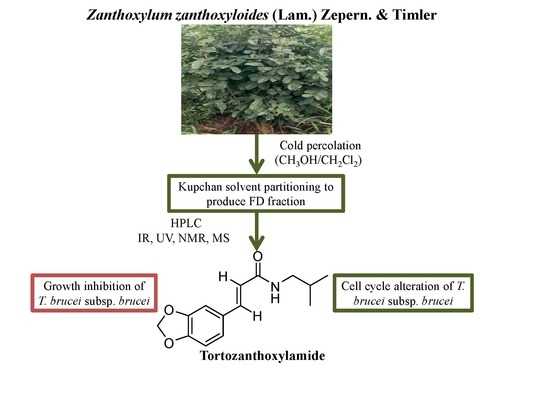
N-(Isobutyl)-3,4-methylenedioxy Cinnamoyl Amide
Abstract
1. Introduction
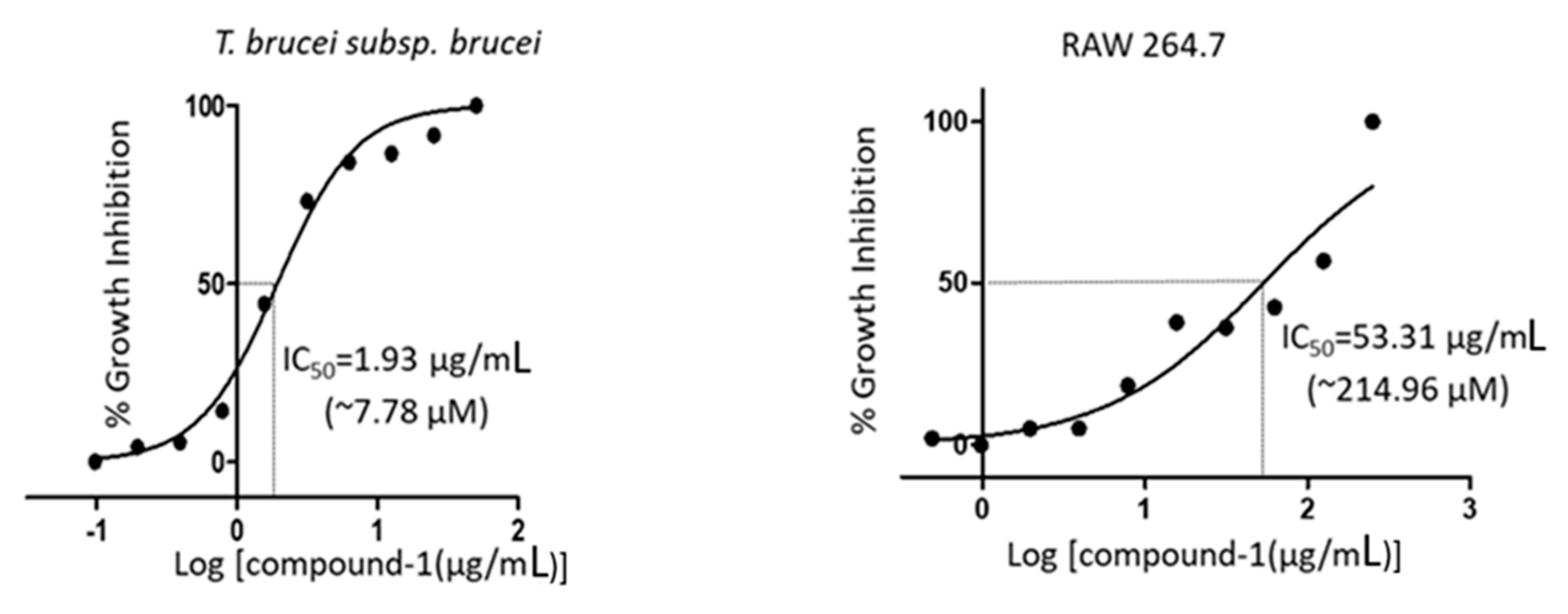
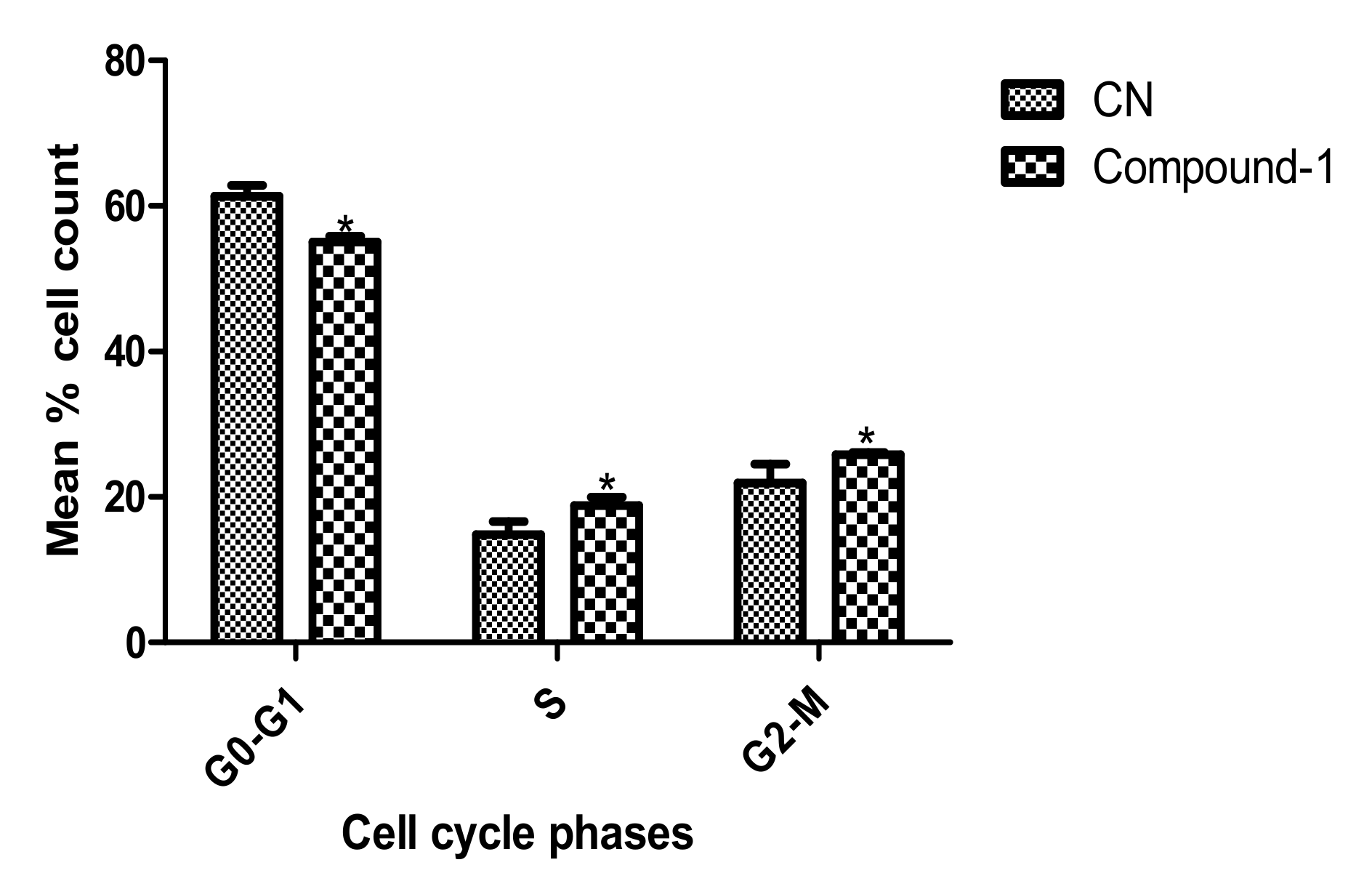
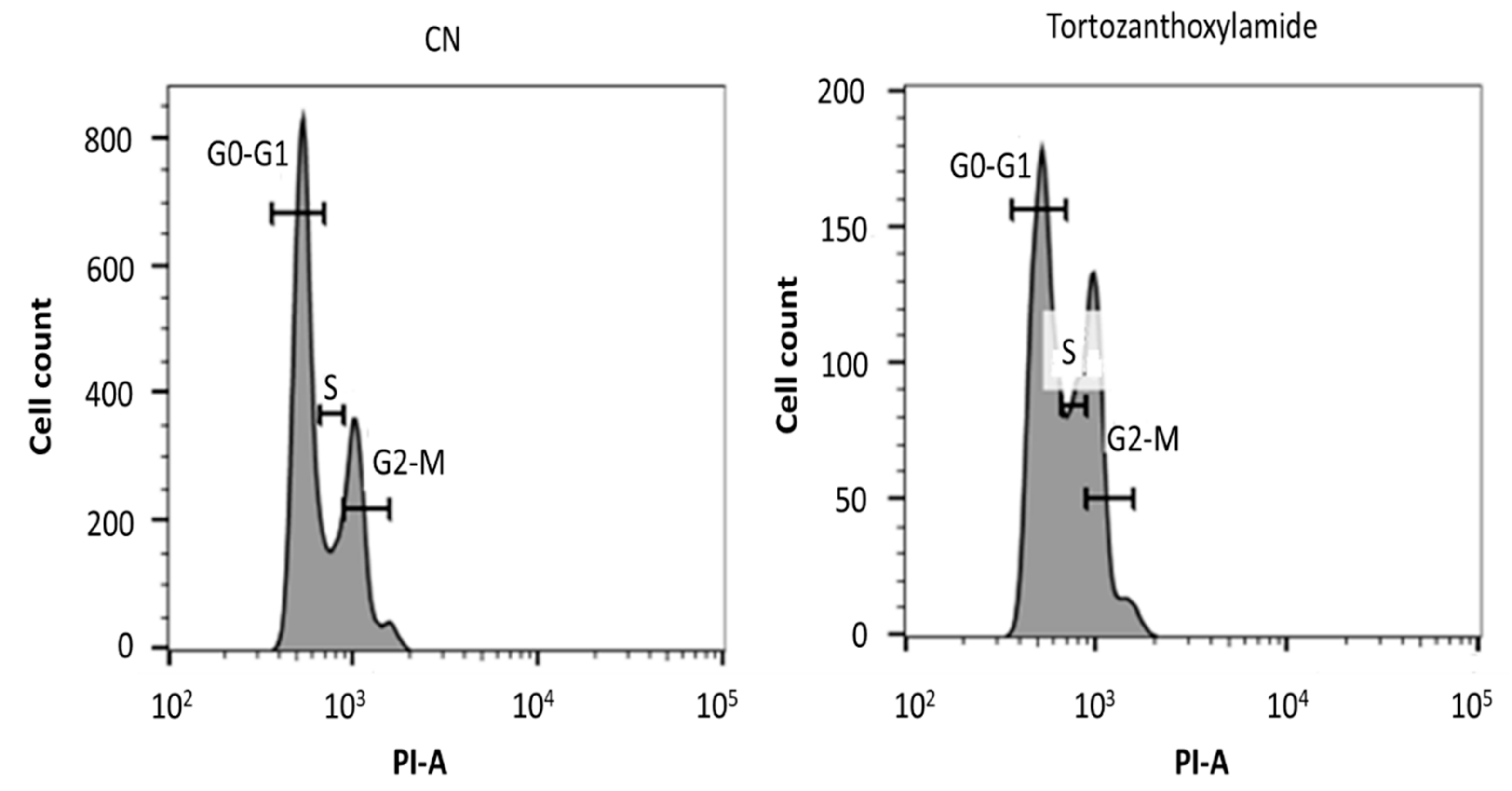
2. Results
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Collection
3.3. Extraction and Purification
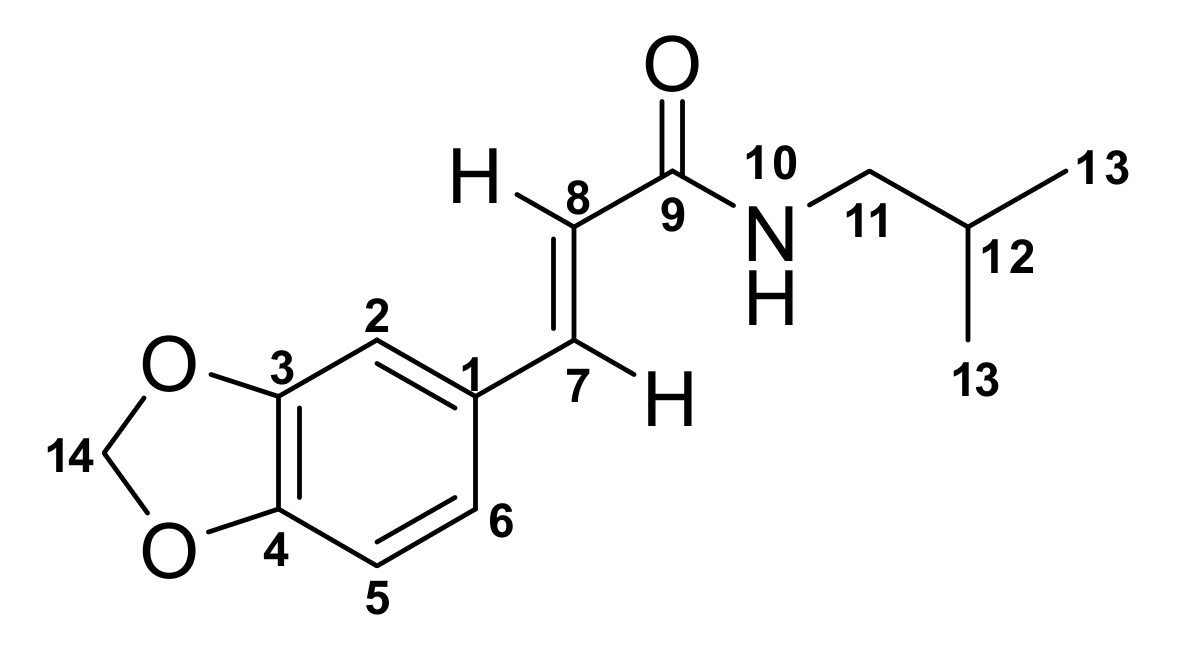
3.4. N-(Isobutyl)-3,4-Methylenedioxy Cinnamoyl Amide (1)
3.5. Culture of Parasites and Mammalian Cell Lines
3.6. Analysis of Cell Viability
3.7. Analysis of Cell Cycle
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simarro, P.P.; Cecchi, G.; Franco, J.R.; Paone, M.; Diarra, A.; Ruiz-Postigo, J.A.; Fèvre, E.M.; Mattioli, R.C.; Jannin, J.G. Estimating and Mapping the Population at Risk of Sleeping Sickness. PLoS Negl. Trop. Dis. 2012, 6, e1859. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.J.; Vezza, L.; Rowan, T.; Hope, J.C. Animal African Trypanosomiasis: Time to Increase Focus on Clinically Relevant Parasite and Host Species. Trends Parasitol. 2016, 32, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Cnops, J.; Magez, S.; De Trez, C. Escape mechanisms of African trypanosomes: Why trypanosomosis is keeping us awake. Parasitology 2015, 142, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D. The development of drugs for treatment of sleeping sickness: A historical review. Parasites Vectors 2010, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.G.; Tait, A.; Turner, C.M. Characterisation of cloned lines of Trypanosoma brucei expressing stable resistance to MelCy and suramin. Acta Trop. 1996, 60, 251–262. [Google Scholar] [CrossRef]
- Matovu, E.; Geiser, F.; Schneider, V.; Maser, P.; Enyaru, J.C.; Kaminsky, R.; Gallati, S.; Seebeck, T. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol. Biochem. Parasitol. 2001, 117, 73–81. [Google Scholar] [CrossRef]
- Baker, N.; Alsford, S.; Horn, D. Genome-wide RNAi screens in African trypanosomes identify the nifurtimox activator NTR and the eflornithine transporter AAT6. Mol. Biochem. Parasitol. 2011, 176, 55–57. [Google Scholar] [CrossRef]
- Barrett, M.P.; Vincent, I.M.; Burchmore, R.J.; Kazibwe, A.J.; Matovu, E. Drug resistance in human African trypanosomiasis. Fut. Microbiol. 2011, 6, 1037–1047. [Google Scholar] [CrossRef]
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Ruiz-Postigo, J.A.; Samo, M.; Jannin, J.G. Monitoring the use of nifurtimox-eflornithine combination therapy (NECT) in the treatment of second stage gambiense human African trypanosomiasis. Res. Rep. Trop. Med. 2012, 3, 93–101. [Google Scholar] [CrossRef]
- Patino, L.O.J.; Prieto, R.J.A.; Cuca, S.L.E. Zanthoxylum Genus as Potential Source of Bioactive Compounds. In Bioactive Compounds in Phytomedicine; Rasooli, I., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 185–218. [Google Scholar]
- Nacoulma, O. Plantes Médicinales et Pratiques Médicales Traditionnelles au Burkina Faso Cas du Plateau Central. Ph.D. Thesis, Université de Ouagadougou, Ouagadougou, Burkina Faso, 1996. [Google Scholar]
- Arbonnier, M. Trees, Shrubs and Lianas of West African Dry Zones; CIRAD Margraf Publishers GMBH MNHN: Paris, France, 2004; p. 573. [Google Scholar]
- Ngassoum, M.B.; Essia-ngang, J.J.; Tatsadjieu, L.N. Antimicrobial study of essential oils of Ocimum gratissimum leaves and Zanthoxylum xanthoxyloides fruits from Cameroon. Fitoterapia 2003, 74, 284–287. [Google Scholar] [CrossRef]
- Ouattara, B.; Angenot, L.; Guissou, P.; Fondu, P.; Dubois, J.; Frédérich, M.; Jansen, O.; Van Heugen, J.C.; Wauters, J.N.; Tits, M. LC/MS/NMR analysis of isomeric divanilloylquinic acids from the root bark of Fagara zanthoxyloides Lam. Phytochem. 2004, 65, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Wouatsa, V.N.A.; Misra, L.; Kumar, S.; Prakash, O.; Khan, F.; Tchoumbougnang, F.; Venkatesh, R.K. Aromatase and glycosyl transferase inhibiting acridone alkaloids from fruits of Cameroonian Zanthoxylum species. Chem. Cent. J. 2013, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Ifarajimi, O.R.; Adewoye, A.T.; Ukam, C.; Udeme, E.E.; Okorie, I.I.; Sakpe, M.S.; Ibrahim, D.R.; Yahaya, Y.A.; Kabir, A.Y.; et al. In vivo antitrypanosomal effects of some ethnomedicinal plants from Nupeland of north central Nigeria. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Ogbadoyi, E.O. Evaluation of Medicinal Plants from Nupeland for Their in vivo Antitrypanosomal Activity. Am. J. Biochem. Mol. Biol. 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Britton, R.W.; Ziegler, M.F.; Sigel, C.W. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973, 38, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Kalia, N.K.; Singh, B.; Sood, R.P. A new amide from Zanthoxylum armatum. J. Nat. Prod. 1999, 62, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.S.; Chen, T.L.; Lin, W.Y.; Tsai, I.L.; Chen, Y.C. Isobutylamides from the fruit of Zanthoxylum integrifoliolum. Phytochemistry 1999, 52, 357–360. [Google Scholar] [CrossRef]
- Vaughan, S.; Gull, K. The structural mechanics of cell division in Trypanosoma brucei. Biochem. Soc. Trans. 2008, 36, 421–424. [Google Scholar] [CrossRef]
- Chou, S.T.; Peng, H.Y.; Chang, C.T.; Yang, J.S.; Chung, H.K.; Yang, S.T.; Wood, W.G.; Chung, J.G. Zanthoxylum ailanthoides Sieb and Zucc. extract inhibits growth and induces cell death through G2/M-phase arrest and activation of apoptotic signals in colo 205 human colon adenocarcinoma cells. Anticancer Res. 2011, 31, 1667–1676. [Google Scholar]
- Dung, T.D.; Chang, H.C.; Binh, T.V.; Lee, M.R.; Tsai, C.H.; Tsai, F.J.; Kuo, W.W.; Chen, L.M.; Huang, C.Y. Zanthoxylum avicennae extracts inhibit cell proliferation through protein phosphatase 2A activation in HA22T human hepatocellular carcinoma cells in vitro and in vivo. Int. J. Mol. Med. 2012, 29, 1045–1052. [Google Scholar]
- Bhatt, V.; Kumar, N.; Sharma, U.; Singh, B. Comprehensive metabolic profiling of Zanthoxylum armatum and Zanthoxylum acanthopodium leaves, bark, flowers and fruits using ultra high performance liquid chromatography. Sep. Sci. Plus 2018, 1, 311–324. [Google Scholar] [CrossRef]
- Goodarzi, S.; Hadjiakhoondi, A.; Yassa, N.; Khanavi, M.; Tofighi, Z. New benzodioxole compounds from the root extract of Astrodaucus persicus. Iran. J. Pharm. Res. 2016, 15, 901–906. [Google Scholar] [PubMed]
- Goodman, C.D.; Austarheim, I.; Mollard, V.; Mikolo, B.; Malterud, K.E.; McFadden, G.I.; Wangensteen, H. Natural products from Zanthoxylum heitzii with potent activity against the malaria parasite. Malar. J. 2016, 15, 481. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Park, G.Y.; Kim, S.H.; Hulme, J.; An, S.S.A. Diminazene aceturate: An antibacterial agent for shiga-toxin-producing Escherichia coli O157:H7. Drug Des. Devel. Ther. 2016, 10, 3363–3378. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, S.; Muleme, H.; Onyilagha, C.; Okeke, E.; Uzonna, J.E. Diminazene aceturate (Berenil) modulates LPS induced pro-inflammatory cytokine production by inhibiting phosphorylation of MAPKs and STAT proteins. Innate Immun. 2014, 20, 760–773. [Google Scholar] [CrossRef]
- Han, D.; Yoon, W.K.; Hyun, C. Cerebellar encephalopathy from diminazene aceturate (beneril) toxicity in a dog. Korean J. Vet. Res. 2014, 54, 193–196. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Bottari, N.B.; Rech, V.C.; Nishihira, V.S.; Oliveira, C.B.; Cargnin, L.P.; Moresco, R.N.; Thomé, G.R.; Schetinger, M.R.C.; Morsch, V.M.; et al. Combination of diminazene aceturate and resveratrol reduces the toxic effects of chemotherapy in treating Trypanosoma evansi infection. Comp. Clin. Path. 2016, 25, 137–144. [Google Scholar] [CrossRef]
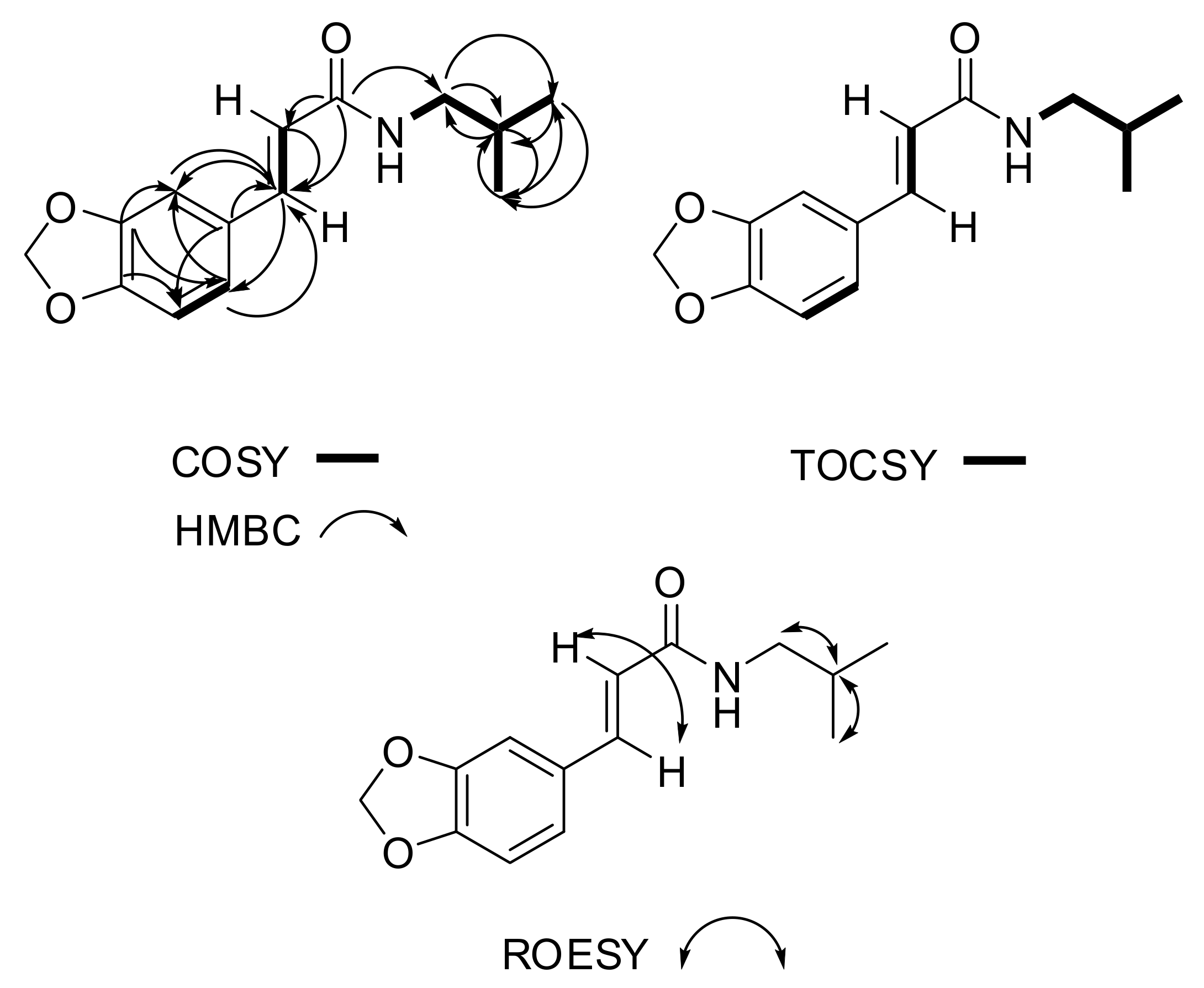
| Signal Number | δc mult | δH mult (J Hz) | 1H-1H COSY | 1H-1H TOCSY | ROESY | HMBC |
|---|---|---|---|---|---|---|
| 1 | 129.4, C | |||||
| 2 | 106.4, CH | 6.94, d (1.7) | C-3, C-7, C-6 | |||
| 3 | 148.9, C | |||||
| 4 | 148.1, C | |||||
| 5 | 108.4, CH | 6.71, d (8.0) | 6 | 6 | C-4, C-1 | |
| 6 | 123.7, CH | 6.90, dd (1.7, 8.0) | 5 | 5 | C-3, C-7, C-2 | |
| 7 | 140.4, CH | 7.49, d (15.5) | 8 | 8 | 8 | C-9, C-1, C-6, C-8, C-2 |
| 8 | 119.2, CH | 6.33, d (15.5) | 7 | 7 | 7 | C-9, C-7, C-1 |
| 9 | 166.4, C | |||||
| 10NH | 2.00 | |||||
| 11 | 47.2, CH2 | 3.17, t (6.7) | 10, 12 | 10, 12, 13 | 12 | C-9, C-12 |
| 12 | 28.7, CH | 1.82, n (6.7) | 11, 13 | 10, 11, 13 | 11, 13 | C-11, C-13, |
| 13 | 20.2, 2CH3 | 0.91, d (6.7) | 12 | 10, 11, 12 | 12 | C-11, C-12, C-13 |
| 14 | 101.4, CH2 | 5.92, s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dofuor, A.K.; Kwain, S.; Osei, E.; Mawuli Tetevi, G.; Okine, L.K.; Ohashi, M.; Gwira, T.M.; Kyeremeh, K. N-(Isobutyl)-3,4-methylenedioxy Cinnamoyl Amide. Molbank 2019, 2019, M1070. https://doi.org/10.3390/M1070
Dofuor AK, Kwain S, Osei E, Mawuli Tetevi G, Okine LK, Ohashi M, Gwira TM, Kyeremeh K. N-(Isobutyl)-3,4-methylenedioxy Cinnamoyl Amide. Molbank. 2019; 2019(3):M1070. https://doi.org/10.3390/M1070
Chicago/Turabian StyleDofuor, Aboagye Kwarteng, Samuel Kwain, Enoch Osei, Gilbert Mawuli Tetevi, Laud Kenneth Okine, Mitsuko Ohashi, Theresa Manful Gwira, and Kwaku Kyeremeh. 2019. "N-(Isobutyl)-3,4-methylenedioxy Cinnamoyl Amide" Molbank 2019, no. 3: M1070. https://doi.org/10.3390/M1070
APA StyleDofuor, A. K., Kwain, S., Osei, E., Mawuli Tetevi, G., Okine, L. K., Ohashi, M., Gwira, T. M., & Kyeremeh, K. (2019). N-(Isobutyl)-3,4-methylenedioxy Cinnamoyl Amide. Molbank, 2019(3), M1070. https://doi.org/10.3390/M1070