Abstract
The title compound, [dicyclohexyl(sulfanylidene)-λ5-phosphanyl]methanol, Cy2P(=S)CH2OH (1), was obtained from the reaction between [Cy2P(CH2OH)2]Cl with one molar equivalent of NaOH and an excess of elemental sulfur (powdered). Characterization was by a single-crystal X-ray structure determination as well as IR, and 1D-NMR (1H, 13C{1H}, 31P{1H}), and 2D-NMR (DEPT-135 and HSQC) spectroscopy, ESI mass spectrometry, and elemental analysis. X-ray crystallography on Compound 1 shows the phosphorus atom to be tetrahedrally coordinated within a non-symmetric C3S donor set but with relatively minor distortions from the ideal geometry. In the molecular packing, hydroxyl-O–H⋯S(thione) hydrogen bonds led to supramolecular helical chains along the b-axis direction.
1. Introduction
Hydroxymethylphosphanes, containing the P-CH2OH functionality, are an important class of compound on account of their reactivity (for example, towards amines) and coordination chemistry [1]. The solid-state structures of compounds containing P-CH2OH groups have also attracted interest for the hydrogen-bonding motifs that can be generated, particularly phosphane chalcogenides, where competition exists between P=S⋯HO and O⋯HO hydrogen bonding. Several tertiary hydroxymethylphosphane sulfide derivatives containing one or two CH2OH groups have been structurally characterized: Fc(CH2)nP(S)(CH2OH)2 where n = 0 [2] or 1 [3], FcCHMeP(S)(CH2OH)2 [4], CyP(S)(CH2OH)2 [5], Ph2P(S)CH2OH [6], and PhCH2P(S)(CH2OH)2 [7]; Fc is ferrocenyl, (η5-C5H5)Fe(η5-C5H4). In this contribution, the synthesis, spectroscopic characterization, and X-ray crystal structure determination of the title Compound 1 and Figure 1, are described. The synthesis of Compound 1 has been described previously but with no characterization data beyond a melting point [8].
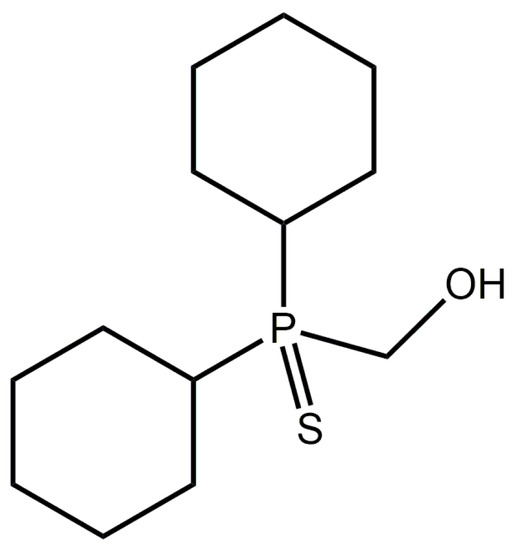
Figure 1.
Chemical diagram for [dicyclohexyl(sulfanylidene)-λ5-phosphanyl]methanol (Compound 1).
2. Results and Discussion
Reaction of the known phosphonium salt [Cy2P(CH2OH)2]Cl [9] with one molar equivalent of sodium hydroxide and an excess of powdered elemental sulfur gave Compound 1 in good (84%) yield, as a colorless microcrystalline solid. This simple, one-pot process, proceeds via the in-situ generation of the intermediate hydroxymethylphosphane Cy2PCH2OH upon reaction of the phosphonium salt with base. Compound 1 was originally prepared by heating Cy2PCH2OH with sulfur in C6H6 or EtOH [8].
A spectroscopic study ensued (IR and 1D-NMR (1H, 13C{1H}, and 31P{1H}) and 2D-NMR (DEPT-135 and HSQC)); see Supplementary Materials for original spectra. The IR spectrum of Compound 1 shows the expected features, including a broad OH band at 3437 cm−1, C-H stretches at 3280, 2930, and 2853 cm−1, and a P=S stretch at 616 cm−1. This P=S stretch is assigned by comparison with related phosphane sulphides, for example, Cy3P=S, which has a P=S stretch at 619 cm−1 [10]. The 1H and 13C{1H} NMR spectra of Compound 1 show the expected features as detailed in Section 3.2. An interesting observation in the 1H NMR spectrum is a single broad resonance at 2.82 ppm assigned to the hydroxyl-proton with the broadness due to intermolecular proton exchange of the hydroxyl-proton; this results in the loss of coupling. This exchange process also can be observed in the 13C{1H} NMR spectrum with the most notable feature being the appearance of two resonances for the phosphorus-bound methylene group. To confirm these observations, 2D-NMR (DEPT-135 and HSQC) experiments were conducted. The DEPT-135 NMR spectra show two negative signals due to the phosphorus-bound methylene group. In addition, HSQC spectra show a correlation between carbon and proton nuclei of the phosphorus-bound methylene group. A single resonance at 62.30 ppm is noted in the 31P{1H} NMR spectrum.
As no X-ray crystal structure determination has been reported for Compound 1, an X-ray crystallographic investigation was undertaken. The molecular structure of Compound 1 is shown in Figure 2 and selected interatomic parameters are included in the figure caption. The phosphorus atom is tetrahedrally coordinated by the thione-S1 atom, the methylene-C1 atom of the hydroxymethyl group and two methine-carbon atoms derived from two cyclohexyl groups. The tetrahedral angles span a relatively narrow range, that is, C1-P1-C2 = 104.72(6)° to S1-P1-C2 = 112.65(4)°, with the widest angles involving the thione-S1 atom. To a first approximation the molecule exhibits mirror symmetry with the P1, S1, C1, and O1 atoms lying on the putative mirror plane. Deviations from the pseudo symmetry are apparent in the S1-P1-C1-O1 torsion angle of −165.67(7)° and in the non-equivalence of the C2/C8-P1-C1-O1 torsion angles of −44.35(10) and 70.76(10)°, respectively.
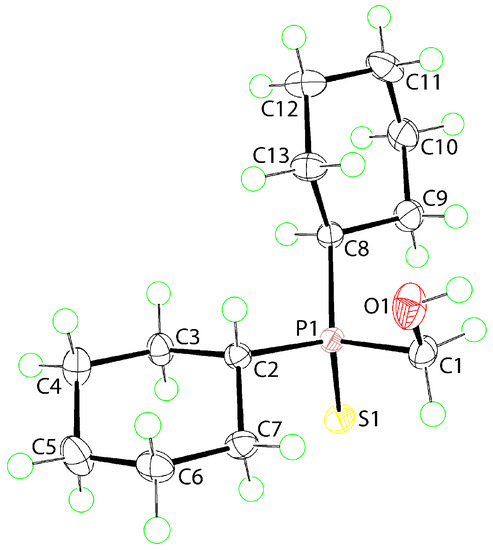
Figure 2.
The molecular structure of Compound 1 showing atom labelling and displacement ellipsoids at the 70% probability level. Selected geometric parameters: P1-S1 = 1.9784(4) Å, P1-C1 = 1.8308(12) Å, P1-C2 = 1.8257(12) Å, P1-C8 = 1.8315(12) Å, S1-P1-C1 = 110.06(4)°, S1-P1-C2 = 112.65(4)°, S1-P1-C8 = 112.49(4)°, C1-P1-C2 = 104.72(6)°, C1-P1-C8 = 108.63(6)°, C2-P1-C8 = 107.93(6)°.
The molecular packing in the crystal of Compound 1 features hydroxyl-O1-H⋯S1(thione) hydrogen bonds which lead to a supramolecular chain, Figure 3a; geometric details characterizing these interactions are given in the figure caption. The chain is aligned along the b-axis direction and, owing to the presence of a 21 screw axis along this direction, has a helical topology. The chains are connected into a three-dimensional architecture with no directional interactions between them, Figure 3b. A Hirshfeld surface analysis was conducted in accordance with established protocols [11] and with the use of Crystal Explorer 17 [12]. The identified hydroxyl-O1-H⋯S1(thione) hydrogen bonds account for 13.2% of the contacts of the overall surface of the molecule whereby non-directional H⋯H contacts contribute almost the balance, that is, 83.7%. The only other surface contacts of note are of the type O⋯H/H⋯O but these occur beyond the sum of the respective van der Waals radii. These observations are entirely consistent with the presence of two bulky, hydrogen-rich cyclohexyl groups in Compound 1.
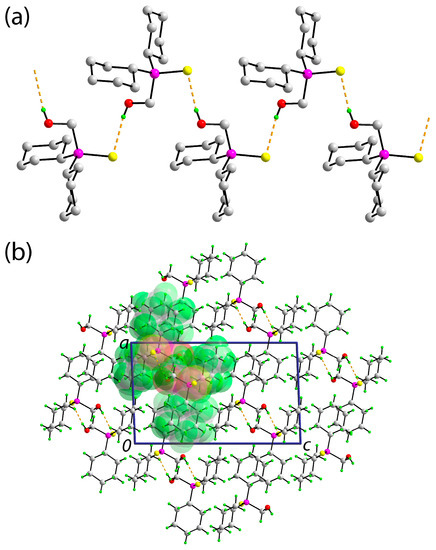
Figure 3.
The molecular packing in the crystal of Compound 1: (a) A view of the helical supramolecular chain along the b-axis sustained by hydroxyl-O-H⋯S(thione) hydrogen bonding [O1-H1o⋯S1i: H1o⋯S1i = 2.378(15) Å, O1⋯S1i 3.1962(10) Å, and angle at H1o = 169.4(14)° for symmetry operation i: 1½ − x, −½ + y, ½ − z] shown as orange dashed lines; non-participating hydrogen atoms have been omitted for reasons of clarity; (b) a view of the unit cell contents shown in projection down the b-axis. One supramolecular chain has been highlighted in space-filling mode.
There are two closely related compounds to Compound 1 in the crystallographic literature. The first of these is the diphenyl analog of Compound 1, Ph2P(=S)CH2OH (2) [6]. Here, the key bond lengths are equivalent to those in Compound 1 and the range of tetrahedral angles, i.e., 105.3(2) to 112.99(12)°, is also narrow. The second closely related structure has one cyclohexyl group in Compound 1 replaced by a second hydroxymethyl group, that is CyP(=S)(CH2OH)2 (3) [5]. In Compound 3, the P1-S1 bond length of 1.9545(10) Å is significantly shorter than the equivalent bonds in Compounds 1 and 2 and the range of tetrahedral angles is significantly wider, i.e., 103.69(10)°, subtended by the methylene-carbon atoms, to 116.56(8)°, for a thione-S-P-C(methylene) angle, indicating the thione-sulfur atom exerts a greater significance upon the molecular geometry in the less sterically congested environment about the phosphorus atom in Compound 3. In the molecular packing of Compound 2, supramolecular chains are formed through the formation of hydroxyl-O-H⋯S(thione) hydrogen bonding as for Compound 1. However, in Compound 2 the chain is propagated by glide symmetry, so the topology of the chain is zigzag. With an additional hydroxyl group in Compound 3, hydroxy-O-H⋯O(hydroxy) hydrogen bonding predominates so that each hydroxyl group functions as a donor and as an acceptor, leading to a linear supramolecular tape comprising edge-shared 10-membered {⋯OCPCOH⋯OH⋯OH} synthons. Highlighting the significant role of the cyclohexyl groups upon surface contacts in Compound 1, H⋯H contacts contribute 51.6% to the Hirshfeld surface of the diphenyl analog, Compound 2.
Finally, Compound 1 was also subjected to an ESI mass spectral analysis; see Supplementary Materials for original spectra. The ESI mass spectrum of Compound 1 in methanol solution showed the ions [1 + Na]+ (calculated m/z 283.13) and [2(1) + Na]+ (calculated m/z 543.26), with the latter dominating at relatively low capillary exit voltages (≤90 V). The source of the sodium cations is adventitious, from the solvent or carried over from the synthesis of the compound (which used NaOH). In addition to these ions, there were a number of relatively low intensity ions. The spectra became simpler when a small quantity of aqueous sodium formate solution was added, which enhanced the formation of the above sodiated ions. Compound 1 also shows a strong [1 + HCO2]− ion in negative ion mode at m/z 305.15 (calculated m/z 305.13), presumably due to the formation of a hydrogen-bonded gas-phase adduct, alongside a number of low-intensity ions. One of these at m/z 229.13 is assigned as the fragment ion [Cy2P(S)]− (calculated m/z 229.12).
In conclusion, X-ray crystallography on Compound 1 reveals a tetrahedrally coordinated phosphorus atom with relatively minor distortions owing to the presence of a non-symmetric C3S donor set. In the crystal, supramolecular helical chains mediated by hydroxyl-O–H⋯S(thione) hydrogen bonds are apparent.
3. Materials and Methods
3.1. General Information
The phosphonium salt [Cy2P(CH2OH)2]Cl was prepared according to the literature method [9], from Cy2PH (Strem Chemical Co., Newburyport, MA, USA), concentrated aqueous formaldehyde, and hydrochloric acid. Methanol (Scharlau Chemie SA, Barcelona, Spain), elemental sulfur powder (Ajax Finechem, Sydney, Australia), and sodium hydroxide (Ajax Finechem) were used as supplied. Melting points were recorded on a Reichert-Jung Thermovar hotstage microscope (Vienna, Austria). Elemental analyses were obtained from the Campbell Microanalytical Laboratory, University of Otago, New Zealand. IR spectra were recorded as KBr disks on a Perkin Elmer Spectrum 100 FTIR spectrometer (Shelton, CT, USA). 1D-NMR (1H and 13C{1H}) and 2D-NMR (DEPT-135 and HSQC) spectra were recorded in CDCl3 solution on a Bruker Ascend 400 MHz NMR (Billerica, MA, USA) spectrometer with chemical shifts relative to tetramethylsilane. The 31P{1H} NMR spectrum was measured on the same instrument relative to 85% H3PO4. ESI mass spectra were recorded on a Bruker MicrOTOF instrument (Bremen, Germany), typically using a capillary exit voltage of 90 V and a Skimmer 1 voltage of 30 V. Assignment of ions was facilitated by comparison of experimental and calculated isotope patterns, the latter obtained using either proprietary instrument software or the open-source software mMass [13].
3.2. Synthesis Cy2P(S)CH2OH (1)
To a solution of [Cy2P(CH2OH)2]Cl (900 mg, 3.05 mmol) in methanol (30 mL) was added powdered elemental sulfur (1.5 g, large excess). A solution of sodium hydroxide (122 mg, 3.04 mmol) in water (5 mL) was added to the vigorously stirred mixture of phosphonium salt and sulfur. The mixture was stoppered and stirred for 48 h. Excess unreacted sulfur was removed by filtration, and the solid washed with an additional 20 mL of methanol. The clear, colorless filtrate was allowed to evaporate at room temperature, producing a colorless microcrystalline solid. The product was filtered, washed with distilled water (5 mL), and dried under vacuum, to give Compound 1 (664 mg, 84%). Crystals suitable for the X-ray diffraction study were obtained from dichloromethane-diethyl ether. M.p. 103–108 °C cf. Lit. 111–113 °C [8]. Found: C 59.95; H 9.72. C13H25OPS requires C 59.97; H 9.69%. 1H NMR {CDCl3}: 3.80 (s, 2H, CH2OH), 2.82 (s, br, 1H, OH), 2.00 (m, 1H, =P-CH), 1.97 (m, 2H, CH2), 1.94 (m, 1H, =P-CH), 1.86 (m, 6H, CH2), 1.73 (m, 2H, CH2), 1.43 (m, 4H, CH2), 1.32 (m, 2H, CH2) 1.24 (m, 4H, CH2). 13C{1H} NMR {CDCl3}: 55.34, 54.86 (CH2-OH), 36.07 (S=P-CH), 35.62 (S=P-CH), 26.51, 26.45 (CH2), 26.38, 26.33 (CH2), 26.01, 25.97 (CH2), 25.73, 25.72 (CH2), 25.59, 25.57 (CH2). 31P {1H} NMR {CDCl3}: 62.30. ESI MS (MeOH, added sodium formate): Positive-ion m/z 283.10 [1 + Na]+ (calculated m/z 283.13), 543.23 [2(1) + Na]+ (calculated m/z 543.26), negative-ion m/z 305.15 [1 + HCO2]− (calculated m/z 305.13).
3.3. Crystallography
Intensity data for Compound 1 were measured at T = 100(2) K on a XtaLAB Synergy Dual AtlasS2 (Rigaku Corporation, Oxford, UK) diffractometer fitted with Cu Kα radiation (λ = 1.54184 Å) using ω-scans so that θmax was 67.1°. Data reduction, including absorption correction, was accomplished with CrysAlis Pro [14]. Of the 17,316 measured reflections, 2512 were unique (Rint = 0.031), and of these, 2376 data satisfied the I ≥ 2σ(I) criterion. The structure was solved by direct methods [15] and refined (anisotropic displacement parameters and C-bound H atoms in the riding model approximation) on F2 [16]. The hydroxyl-H atom was located from a difference map and refined with O-H restrained to 0.84 ± 0.01 Å. A weighting scheme of the form w = 1/[σ2(Fo2) + (0.032P)2 + 0.629P] was introduced, where P = (Fo2 + 2Fc2)/3). Based on the refinement of 148 parameters, the final values of R and wR (all data) were 0.024 and 0.064, respectively. The molecular structure diagram was generated with ORTEP for Windows [17] and the packing diagram using DIAMOND [18].
Crystal data for C13H25OPS (1): M = 260.36, monoclinic, P21/n, a = 9.9253(1), b = 8.70880(10), c = 16.2749(2) Å, β = 92.1500(10)°, V = 1405.77(3) Å3, Z = 4, Dx = 1.230 g cm−3, F(000) = 568, and μ = 2.942 mm−1. CCDC deposition number: 1918283.
Supplementary Materials
The following are available online: IR, 1D-NMR 1H, 13C{1H}, and 31P{1H}, and 2D-NMR (DEPT-135 and HSQC) spectra, ESI mass spectra, and crystallographic data for Compound 1 in crystallographic information file (CIF) format. CCDC 1918283 also contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html.
Author Contributions
Synthesis was performed by W.H. Mass spectroscopic characterization was carried out by W.H., NMR was by A.H.S.A., and X-ray crystallography by O.C.O. and E.R.T.T. The manuscript was written by W.H. and E.R.T.T.
Funding
The APC was funded by Sunway University Sdn Bhd. The University of Waikato and Sunway University Sdn Bhd (Grant. no. STR-RCTR-RCCM-001-2019) are thanked for financial support of this work.
Acknowledgments
We thank Stacey J. Scown (University of Waikato) for the initial synthesis of a sample of Compound 1. The X-ray crystallography laboratory at Sunway University is thanked for the X-ray intensity data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- James, B.R.; Lorenzini, F. Developments in the chemistry of tris(hydroxymethyl)phosphine. Coord. Chem. Rev. 2010, 254, 420–430. [Google Scholar] [CrossRef]
- Henderson, W.; Alley, S.R. Ferrocenyl hydroxymethylphosphines (η5-C5H5)Fe[η5-C5H4P(CH2OH)2] and 1,1′-[Fe{η5-C5H4P(CH2OH)2}2] and their chalcogenide derivatives. J. Organomet. Chem. 2002, 658, 181–190. [Google Scholar] [CrossRef]
- Goodwin, N.J.; Henderson, W.; Nicholson, B.K.; Sarfo, J.K.; Fawcett, J.; Russell, D.R. Synthesis and reactivity of the ferrocene-derived phosphine [Fe(η-C5H5){η-C5H4CH2P(CH2OH)2}]. J. Chem. Soc. Dalton Trans. 1997, 4377–4384. [Google Scholar] [CrossRef]
- Ramakrishna, T.V.V.; Elias, A.J.; Vij, A. Synthesis and reactions of the ferrocene derived hydroxymethyl phosphine FcCH(CH3)P(CH2OH)2 and its sulfide: Crystal structures of [FcCH(CH3)P(S)R2] R=CH2OH, CH2CH2CN and FcCH(CH3)P(S)(CH2O)2PPh (Fc=ferrocenyl). J. Organomet. Chem. 2000, 602, 125–132. [Google Scholar] [CrossRef]
- Gonschorowsky, M.; Merz, K.; Driess, M. Cyclohexylbis(hydroxymethyl)phosphane: A hydrophilic phosphane capable of forming novel hydrogen-bonding networks. Eur. J. Inorg. Chem. 2006, 455–463. [Google Scholar] [CrossRef]
- Goodwin, N.J.; Henderson, W.; Nicholson, B.K. Hydrogen bonding in hydroxymethylphosphine chalcogenides. Inorg. Chim. Acta 2002, 335, 113–118. [Google Scholar] [CrossRef]
- Griffiths, D.V.; Groombridge, H.J.; Salt, M.C. Investigations into the oxidative stability of hydroxymethyl- and bis(hydroxymethyl)-phosphines. Phosphorus, Sulfur, Silicon, Relat. Elem. 2008, 183, 473–478. [Google Scholar] [CrossRef]
- Hellmann, H.; Bader, J.; Birkner, H.; Schumacher, O. Hydroxymethylphosphines, hydroxymethylphosphonium salts, and chloromethylphosphonium salts. Justus Liebigs Ann. Chem. 1962, 659, 49–63. [Google Scholar] [CrossRef]
- Fawcett, J.; Hoye, P.A.T.; Kemmitt, R.D.W.; Law, D.J.; Russell, D.R. Synthesis of bis(phosphinomethyl)amines via bis(hydroxymethyl)phosphonium salts. Isolation of 9,9-bis(hydroxymethyl)-9-phosphoniabicyclo[3.3.1]nonane hydrogen sulfate and chloride salts, and the crystal structures of [PPh2(CH2OH)2]+ Cl− and [(C6H11)2PCH2]2NCHMePh. J. Chem. Soc. Dalton Trans. 1993, 2563–2568. [Google Scholar] [CrossRef]
- Zingaro, R.A. Phosphine sulfides and selenides: The phosphorus-sulfur and phosphorus-selenium stretching frequencies. Inorg. Chem. 1963, 2, 192–196. [Google Scholar] [CrossRef]
- Tan, S.L.; Jotani, M.M.; Tiekink, E.R.T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. E 2019, 75, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; Mckinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer; v17; The University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Strohalm, M.; Hassman, M.; Košata, B.; Kodíček, M. mMass data miner: An open source alternative for mass spectrometric data analysis. Rapid Commun. Mass Spectrom. 2008, 22, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlis PRO.; Rigaku Corporation: Oxford, UK, 2017. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. DIAMOND.; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).