Synthesis and Crystal Structure of A Pyrithione Derivative: Bis{2-[(1-oxidopyridin-2-yl)sulfanyl]-4,5-dihydro-1H-imidazol-3-ium} tetrachlorocuprate(2-)
Abstract
:1. Introduction
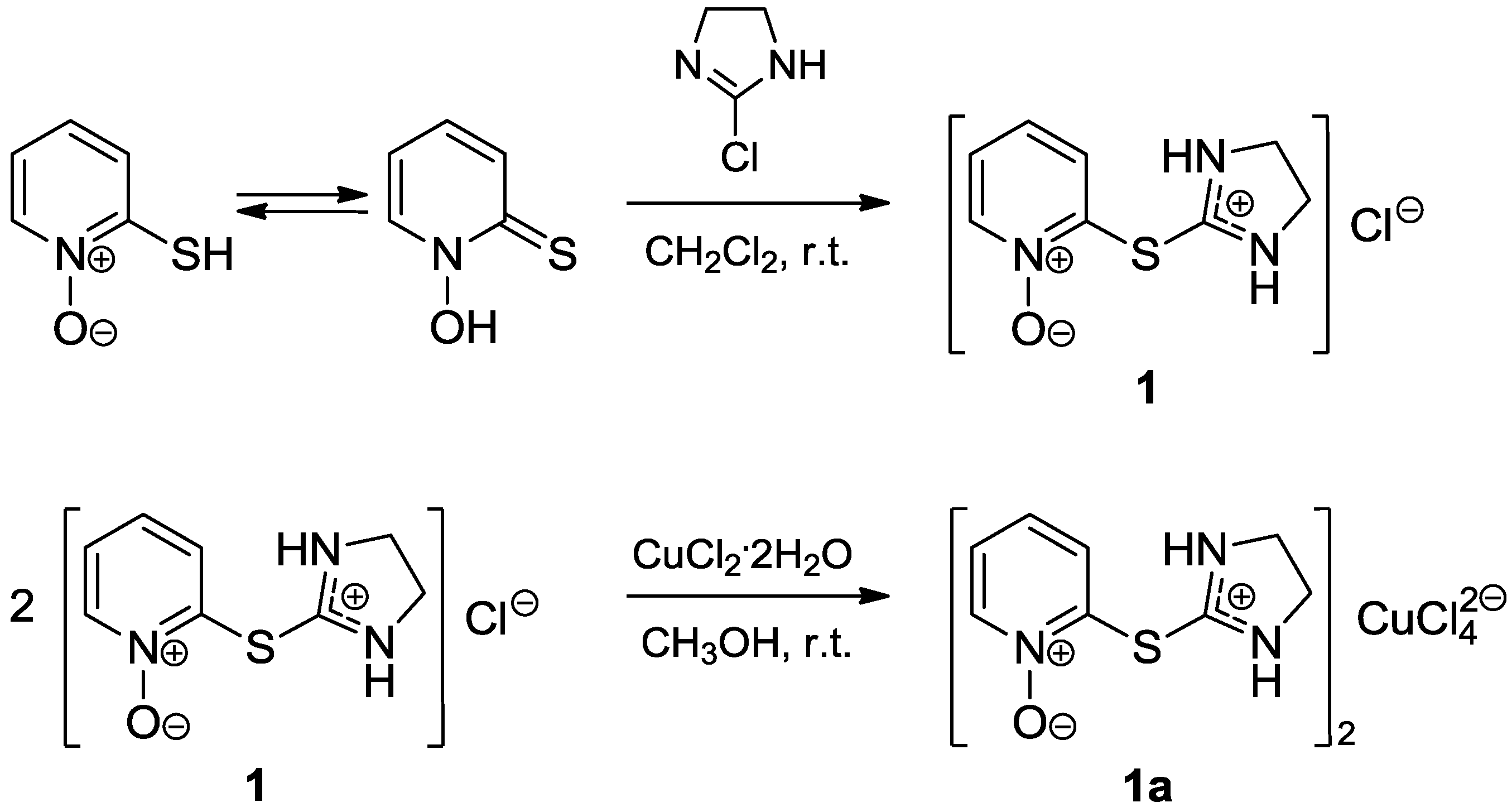
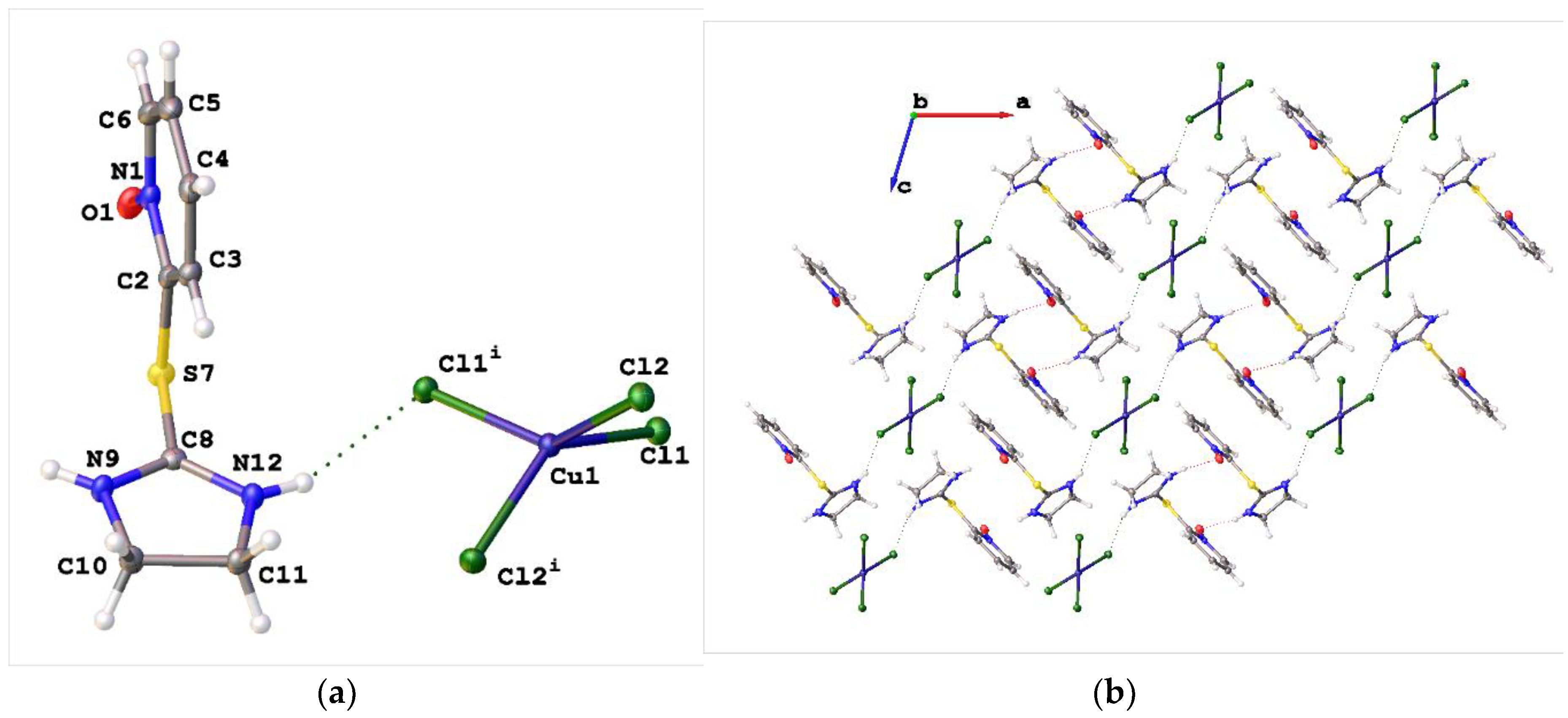
2. Results and Discussion
3. Materials and Methods
3.1. General Methods and Physical Measurements
3.2. Synthesis of 2-[(4,5-Dihydro-1H-imidazol-2-yl)thio]pyridine 1-oxide hydrochloride (1)
3.3. Synthesis of Bis{2-[(1-oxidopyridin-2-yl)sulfanyl]-4,5-dihydro-1H-imidazol-3-ium} tetrachlorocuprate(2-) (1a)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.C.; Hu, Y.; Wu, D.; Weng, L.; Kang, B. Syntheses and electrochemistry of some transition metal complexes with 2-mercaptopyridine N-oxide and crystal structure of bis(2-mercaptopyridine N-oxide)nickel(II). Polyhedron 1991, 10, 2651–2657. [Google Scholar] [CrossRef]
- Lobana, T.S.; Singh, R. Chemistry of pyridinethiols and related ligands-4. Complexes of bis(pyridine-2-thiolato- or 1-oxopyridine-2-thione) ruthenium(II) with bis(diphenylphosphino)alkanes. Polyhedron 1995, 14, 907–912. [Google Scholar] [CrossRef]
- Barnett, B.L.; Kretschmar, H.C.; Hartman, F.A. Structural characterization of bis(N-oxopyridine-2-thionato)zinc(II). Inorg. Chem. 1977, 16, 1834–1838. [Google Scholar] [CrossRef]
- Niu, D.-Z.; Yao, L.; Min, X.; Zou, H. Crystal structure of cis-bis [1-hydroxypyridine-2(1H)-thionato-S,O]copper(II), Cu(C5H4NOS)2. Z. Kristall. NCS 2011, 226, 527–528. [Google Scholar] [CrossRef]
- Jones, R.A.; Katritzky, A.R. Tautomeric pyridines. Part I. Pyrid-2- and -4-thione. J. Chem. Soc. 1958, 3610–3613. [Google Scholar] [CrossRef]
- Jones, R.A.; Katritzky, A.R. N-oxides and related compounds. Part XVII. The tautomerism of mercapto- and acylamino-pyridine 1-oxides. J. Chem. Soc. 1960, 2937–2942. [Google Scholar] [CrossRef]
- Daly, A.M.; Mitchell, E.G.; Sanchez, D.A.; Block, E.; Kukolich, S.G. Microwave spectra and gas phase structural parameters for N-hydroxypyridine-2(1H)-thione. J. Phys. Chem. A 2011, 115, 14526–14530. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Bernstein, J.; Losee, K.; Lott, W.A. Analogs of aspergillic acid. IV. Substituted 2-bromopyridine-N-oxides and their conversion to cyclic thiohydroxamic acids 1. J. Am. Chem. Soc. 1950, 72, 4362–4364. [Google Scholar] [CrossRef]
- Chandler, C.J.; Segel, I.H. Mechanism of the antimicrobial action of pyrithione: Effects on membrane transport, ATP levels, and protein synthesis. Antimicrob. Agents Chemother. 1978, 14, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.R.; Bacon, R.A.; Shah, R.; Mizoguchi, H.; Tosti, A. Therapeutic efficacy of anti-dandruff shampoos: A randomized clinical trial comparing products based on potential zinc pyrithione and zinc pyrithione/climbazole. Int. J. Cosmet. Sci. 2013, 35, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Brooks, L.; Ebsworth-Mojica, K.; Didione, L.; Wucher, B.; Dewhurst, S.; Krysan, D.; Dunman, P.M.; Wozniak, R.A.F. Zinc pyrithione improves the antibacterial activity of silver sulfadiazine ointment. mSphere 2016, 1, e00194-16. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Kaufman, C.W.; Hobbs, P.; Jardas, J.J.; Ruggiero, M.A.; Arif, S. Process for Preparing Copper Pyrithione. U.S. Patent 005540860A, 30 July 1996. [Google Scholar]
- Chas. Pfizer & Co., Inc. Basic Thio-Ethers and Their Preparation. GB Patent 847 701; BE 565868, 1960. [Google Scholar]
- Conover, L.H.; English, A.R.; Larrabee, C.E. Patent: Substituted Pyridine and Quinoline N-oxides and Process for Producing the Same. U.S. Patent 2921073, 12 January 1960. [Google Scholar]
- Strandskov, F.B.; Bockelmann, J.B.; Carroll, V.J. Synthetic Organic Chemical Preservative for Beer. U.S. Patent 3048488, 7 August 1962. [Google Scholar]
- Chang, Z.C.; Jiang, Q.; Xu, B.X.; Tu, S.Z.; Ma, Z.Z.; Zhu, P.; Yang, Z.H. Synthesis of S-(2-pyridyl-N-oxide)-N-substituted or -N,N’-disubstituted isothiourea hydrobromides and their antimicrobial activity. Acta Pharm. Sin. 1995, 30, 263–268. [Google Scholar]
- Yamaguchi, T.; Sakaguchi, J.; Mitsui, S.; Hirai, H.; Takeuchi, Y. N-Oxy-2-pyridylisothiourea Derivatives as Bactericides and Fungicides. JP 51133425, 19 November 1976. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–343. [Google Scholar] [CrossRef]
- CrysAlis Pro Software; ver. 1.171.33; Oxford Diffraction Ltd.: Oxfordshire, UK, 2009.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2014, C71, 3–8. [Google Scholar]
- Trani, A.; Bellasio, E. Synthesis of 2-chloro-2-imidazoline and its reactivity with aromatic amines, phenols, and thiophenols. J. Heterocycl. Chem. 1974, 11, 257–261. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balewski, Ł.; Sączewski, F.; Gdaniec, M. Synthesis and Crystal Structure of A Pyrithione Derivative: Bis{2-[(1-oxidopyridin-2-yl)sulfanyl]-4,5-dihydro-1H-imidazol-3-ium} tetrachlorocuprate(2-). Molbank 2019, 2019, M1067. https://doi.org/10.3390/M1067
Balewski Ł, Sączewski F, Gdaniec M. Synthesis and Crystal Structure of A Pyrithione Derivative: Bis{2-[(1-oxidopyridin-2-yl)sulfanyl]-4,5-dihydro-1H-imidazol-3-ium} tetrachlorocuprate(2-). Molbank. 2019; 2019(2):M1067. https://doi.org/10.3390/M1067
Chicago/Turabian StyleBalewski, Łukasz, Franciszek Sączewski, and Maria Gdaniec. 2019. "Synthesis and Crystal Structure of A Pyrithione Derivative: Bis{2-[(1-oxidopyridin-2-yl)sulfanyl]-4,5-dihydro-1H-imidazol-3-ium} tetrachlorocuprate(2-)" Molbank 2019, no. 2: M1067. https://doi.org/10.3390/M1067
APA StyleBalewski, Ł., Sączewski, F., & Gdaniec, M. (2019). Synthesis and Crystal Structure of A Pyrithione Derivative: Bis{2-[(1-oxidopyridin-2-yl)sulfanyl]-4,5-dihydro-1H-imidazol-3-ium} tetrachlorocuprate(2-). Molbank, 2019(2), M1067. https://doi.org/10.3390/M1067




