α-d-Glucopyranosyl-(1→2)-[6-O-(l-tryptophanyl)-β-d-fructofuranoside]
Abstract
1. Introduction
2. Results
3. Experimental Section
3.1. General Experimental Procedures
3.2. Mangrove Plant Sample Collection
3.3. Isolation and Purification of the Endophytic Mycobacterium Strain
3.4. Fermentation
3.4.1. Extraction and Purification
3.4.2. α-d-Glucopyranosyl-(1→2)-[6-O-(l-tryptophanyl)-β-d-fructofuranoside] (1)
3.4.3. Acid Hydrolysis of Compound 1
3.5. Bioassay Reagents
3.5.1. Compound Preparation for Bioassay
3.5.2. Cell Culture
3.5.3. In Vitro Viability Test for Trypanosome Parasites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, F. Carbohydrate drugs: Current status and development prospect. Drug Discov. Ther. 2015, 9, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Tulsi: A holy plant with high medicinal and therapeutic value. IJGP 2017, 11, S1–S12. [Google Scholar]
- Wu, C.Y.; Ke, Y.; Zeng, Y.F.; Zhang, Y.W.; Yu, H.J. Anticancer activity of Astragalus polysaccharide in human non-small cell lung cancer cells. Cancer Cell Int. 2017, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Kataoka, T.; Ando, T. The use of lentinan for treating gastric cancer. Anticancer Agents Med. Chem. 2017, 13, 681–688. [Google Scholar] [CrossRef]
- Jiang, R.Z.; Wang, Y.; Luo, H.M.; Cheng, Y.Q.; Chen, Y.H.; Gao, Y.; Gao, Q.P. Effect of the molecular mass of Tremella polysaccharides on accelerated recovery from cyclophosphamide-induced leucopenia in rats. Molecules 2012, 17, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wei, W.; Wang, N. Tremella polysaccharides inhibit cellular apoptosis and autophagy induced by Pseudomonas aeruginosa lipopolysaccharide in A549 cells through sirtuin 1 activation. Oncol. Lett. 2018, 15, 9609–9616. [Google Scholar] [PubMed]
- Silver, S.A.; Harel, Z.; Perl, J. Practical considerations when prescribing icodextrin: A narrative review. Am. J. Nephrol. 2014, 39, 515–527. [Google Scholar] [CrossRef]
- Kumagai, K.; Shirabe, S.; Miyata, N.; Murata, M.; Yamauchi, A.; Kataoka, Y.; Niwa, M. Sodium pentosan polysulfate resulted in cartilage improvement in knee osteoarthritis-An open clinical trial. BMC Clin. Pharmacol. 2010, 10, 7. [Google Scholar] [CrossRef]
- Lee-Robichaud, H.; Thomas, K.; Morgan, J.; Nelson, R.L. Cochrane review: Lactulose versus polyethylene glycol for chronic constipation. Evid. Based Child Health 2011, 6, 824–864. [Google Scholar] [CrossRef]
- Ulbrich, W.; Lamprecht, A. Targeted drug-delivery approaches by nanoparticulate carriers in the therapy of inflammatory diseases. J. Royal Soc. Interface 2009, 7, s55–S66. [Google Scholar] [CrossRef]
- Kojima, K.; Tsujimoto, T.; Fujii, H.; Morimoto, T.; Yoshioka, S.; Kato, S.; Yasuhara, Y.; Aizawa, S.; Sawai, M.; Makutani, S.; et al. Pneumatosis cystoides intestinalis induced by the alpha-glucosidase inhibitor miglitol. IM 2010, 49, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Manna, L.; Reale, S.; Vitale, F.; Picillo, E.; Pavone, L.M.; Gravino, A.E. Real-time PCR assay in Leishmania-infected dogs treated with meglumine antimoniate and allopurinol. Vet. J. 2008, 177, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Elshahawi, S.I.; Shaaban, K.A.; Kharel, M.K.; Thorson, J.S. A comprehensive review of glycosylated bacterial natural products. Chem. Soc. Rev. 2015, 44, 7591–7697. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Lv, M.; Hu, J.; Huang, K.; Xu, H. Glycosylation and activities of natural products. Mini Rev. Med. Chem. 2016, 16, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Malmierca, M.G.; González-Montes, L.; Pérez-Victoria, I.; Sialer, C.; Braña, A.F.; García Salcedo, R.; Martín, J.; Reyes, F.; Méndez, C.; Olano, C.; et al. Searching for glycosylated natural products in actinomycetes and identification of novel macrolactams and angucyclines. Front. Microbiol. 2018, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.P.; Parajuli, P.; Sohng, J.K. Metabolic engineering of glycosylated polyketide biosynthesis. Emerg. Top. Life Sci. 2018, 2, 389–403. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhaya, K.; Mishra, K.B.; Shukla, A.K.; Tripathi, R.P.; Tiwari, V.K. Carbohydrate-Based Therapeutics: A Frontier in Drug Discovery and Development. Stud. Nat. Prod. Chem. 2016, 49, 307–361. [Google Scholar]
- Viuff, A.H.; Besenbacher, L.M.; Kamori, A.; Jensen, M.T.; Kilian, M.; Kato, A.; Jensen, H.H. Stable analogues of nojirimycin–synthesis and biological evaluation of nojiristegine and manno-nojiristegine. Org. Biomol. Chem. 2015, 13, 9637–9658. [Google Scholar] [CrossRef]
- Schnell, O.; Weng, J.; Sheu, W.H.H.; Watada, H.; Kalra, S.; Soegondo, S.; Yamamoto, N.; Rathod, R.; Zhang, C.; Grzeszczak, W. Acarbose reduces body weight irrespective of glycemic control in patients with diabetes: Results of a worldwide, non-interventional, observational study data pool. J. Diabetes Complicat. 2016, 30, 628–637. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Bhutani, J.; O’Keefe, J.H. Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2015, 2. [Google Scholar] [CrossRef]
- Mahajan, G.B.; Balachandran, L. Antibacterial agents from actinomycetes-a review. Front. Biosci. 2012, 4, 240–253. [Google Scholar] [CrossRef]
- Ishii, H.; Hirai, K.; Sugiyama, K.; Nakatani, E.; Kimura, M.; Itoh, K. Validation of a Nomogram for Achieving Target Trough Concentration of Vancomycin: Accuracy in Patients With Augmented Renal Function. Ther. Drug Monit. 2018, 40, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Martinez-Abad, A.; Gimeno-Alcañiz, J.V.; Ocio, M.J.; Lagaron, J.M. Controlled delivery of gentamicin antibiotic from bioactive electrospun polylactide-based ultrathin fibers. Adv. Eng. Mater. 2012, 14, B112–B122. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Digoxin, HIF-1, and cancer. Proc. Natl. Acad. Sci. USA 2009, 106, E26–E26. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, T.; Mase, N.; Sasaki, H.; Teruya, T.; Hasegawa, S.I.; Cha, B.Y.; Yagasaki, K.; Suenaga, K.; Nagai, K.; Woo, J.T. Biselyngbyaside, isolated from marine cyanobacteria, inhibits osteoclastogenesis and induces apoptosis in mature osteoclasts. J. Cell. Biochem. 2012, 113, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Neuhof, T.; Schmieder, P.; Preussel, K.; Dieckmann, R.; Pham, H.; Bartl, F.; von Döhren, H.; Hassallidin, A. A glycosylated lipopeptide with antifungal activity from the cyanobacterium Hassallia sp. J. Nat. Prod 2005, 68, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Neuhof, T.; Schmieder, P.; Seibold, M.; Preussel, K.; von Döhren, H. Hassallidin B-Second antifungal member of the Hassallidin family. Bioorg. Med. Chem. Lett. 2006, 16, 4220–4222. [Google Scholar] [CrossRef] [PubMed]
- Perez-Zuniga, F.J.; Seco, E.M.; Cuesta, T.; Degenhardt, F.; Rohr, J.; Vallin, C.; Iznaga, Y.; Perez, M.E.; Gonzalez, L.; Malpartida, F. CE-108, a new macrolide tetraene antibiotic. J. Antibiot. 2004, 57, 197–204. [Google Scholar]
- Lachaud, L.; Bourgeois, N.; Plourd, M.; Leproho, P.; Bastien, P.; Ouellette, M. Parasite susceptibility to amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clin. Infect. Dis. 2009, 48, e16–e22. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, M.; Singh, R.K. Leishmaniasis: Current status of available drugs and new potential drug targets. Asian Pac. J. Trop. Med. 2012, 5, 485–497. [Google Scholar] [CrossRef]
- Fernández, M.M.; Malchiodi, E.L.; Algranati, I.D. Differential effects of paromomycin on ribosomes of Leishmania mexicana and mammalian cells. Antimicrob. Agents Chemother. 2011, 55, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Fosso, M.Y.; Li, Y.; Garneau-Tsodikova, S. New trends in the use of aminoglycosides. MedChemComm 2014, 5, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Li, Y.X.; Dewapriya, P.; Ryu, B.; Kim, S.K. Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia. BMB Rep. 2013, 46, 398. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.H.; Viac, J.; Werling, D.; Rème, C.A.; Gatto, H. Role of sugars in surface microbe–host interactions and immune reaction modulation. Vet. Dermatol. 2007, 18, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Flannery, A.; Gerlach, J.; Joshi, L.; Kilcoyne, M. Assessing bacterial interactions using carbohydrate-based microarrays. Microarrays 2015, 4, 690–713. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Pomin, V. Marine carbohydrate-based compounds with medicinal properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef]
- Clark, G.F. The role of carbohydrate recognition during human sperm–egg binding. Hum. Reprod. 2013, 28, 566–577. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Wang, L.; Song, L. Pathogen-derived carbohydrate recognition in molluscs immune defense. Int. J. Mol. Sci. 2018, 19, 721. [Google Scholar] [CrossRef]
- Feinberg, H.; Jégouzo, S.A.; Rowntree, T.J.; Guan, Y.; Brash, M.A.; Taylor, M.E.; Weis, W.I.; Drickamer, K. Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J. Biol. Chem. 2013, 288, 28457–28465. [Google Scholar] [CrossRef]
- Glavey, S.V.; Huynh, D.; Reagan, M.R.; Manier, S.; Moschetta, M.; Kawano, Y.; Roccaro, A.M.; Ghobrial, I.M.; Joshi, L.; O’Dwyer, M.E. The cancer glycome: Carbohydrates as mediators of metastasis. Blood Rev. 2015, 29, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Camas, M.; Camas, A.S.; Kyeremeh, K. Extracellular Synthesis and Characterization of Gold Nanoparticles Using Mycobacterium sp. BRS2A-AR2 Isolated from the Aerial Roots of the Ghanaian Mangrove Plant, Rhizophora racemosa. Indian J. Microbiol. 2018, 58, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, P.B. The Botany of Mangroves, 2nd ed.; Cambridge University Press: Cambridge, UK, 1994; pp. 326–336. [Google Scholar]
- Hughes, R.H. A Directory of African Wetlands; International Union of Conservation of Nature (IUCN): Gland, Switzerland; Cambridge, UK, 1992; pp. 508–510. [Google Scholar]
- Duke, N.C.; Allen, J.A. Atlantic–East Pacific Red Mangroves: Rhizophora Mangle, R. Samoensis, R. Racemosa, R. X Harrisonii; Permanent Agriculture Resources (PAR): Holualoa, HI, USA, 2006; pp. 1–18. [Google Scholar]
- Beatriz, B.M.; Barreto, E. First report of Rhizophora racemosa Meyer (Rhizophoraceae) in the mangrove forests of the Venezuelan Caribbean coast. Interciencia 2012, 37, 133–137. [Google Scholar]
- Levasseur, A.; Asmar, S.; Robert, C.; Drancourt, M. Draft genome sequence of Mycobacterium houstonense strain ATCC 49403T. Genome Announc. 2016, 4. [Google Scholar] [CrossRef]
- Hennessee, C.T.; Seo, J.S.; Alvarez, A.M.; Li, Q.X. Polycyclic aromatic hydrocarbon-degrading species isolated from Hawaiian soils: Mycobacterium crocinum sp. nov., Mycobacterium pallens sp. nov., Mycobacterium rutilum sp. nov., Mycobacterium rufum sp. nov. and Mycobacterium aromaticivorans sp. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.E. Epidemiology, pathology, immunology and diagnosis of bovine farcy: A review. Prev. Vet. Med. 2012, 105, 1–9. [Google Scholar] [CrossRef]
- McGettrick, A.F.; Corcoran, S.E.; Barry, P.J.; McFarland, J.; Crès, C.; Curtis, A.M.; Franklin, E.; Corr, S.C.; Mok, K.H.; Cummins, E.P.; et al. Trypanosoma brucei metabolite indolepyruvate decreases HIF-1α and glycolysis in macrophages as a mechanism of innate immune evasion. Proc. Natl. Acad. Sci. USA 2016, 113, E7778–E7787. [Google Scholar] [CrossRef]
- Marchese, L.; Nascimento, J.; Damasceno, F.; Bringaud, F.; Michels, P.; Silber, A. The uptake and metabolism of amino acids, and their unique role in the biology of pathogenic trypanosomatids. Pathogens 2018, 7, 36. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Britton, R.W.; Ziegler, M.F.; Sigel, C.W. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973, 38, 178–179. [Google Scholar] [CrossRef]
- Yabu, Y.; Minagawa, N.; Kita, K.; Nagai, K.; Honma, M.; Sakajo, S.; Koide, T.; Ohta, N.; Yoshimoto, A. Oral and intraperitoneal treatment of Trypanosoma brucei brucei with a combination of ascofuranone and glycerol in mice. Parasitol. Int. 1998, 47, 131–137. [Google Scholar] [CrossRef]
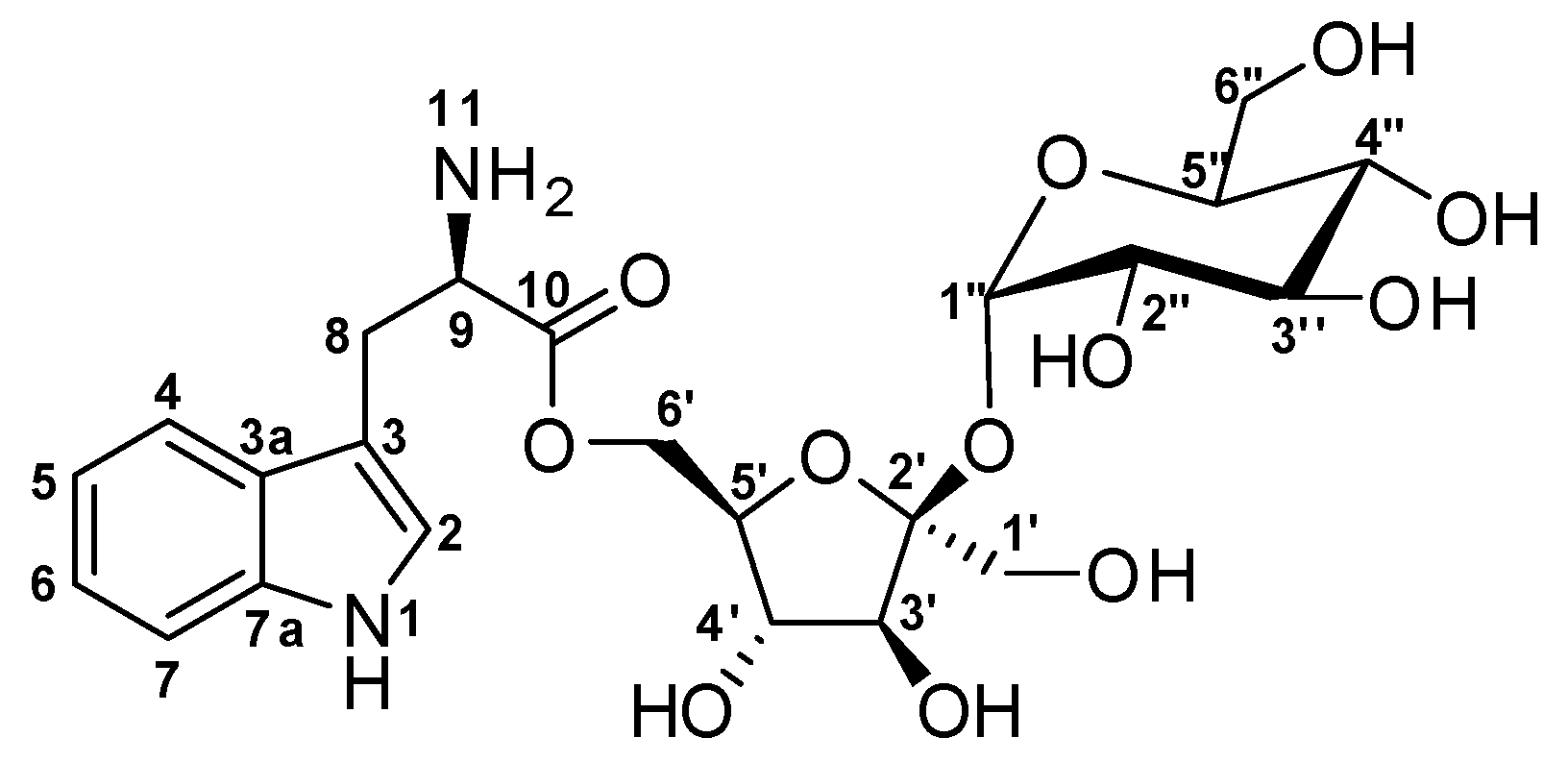
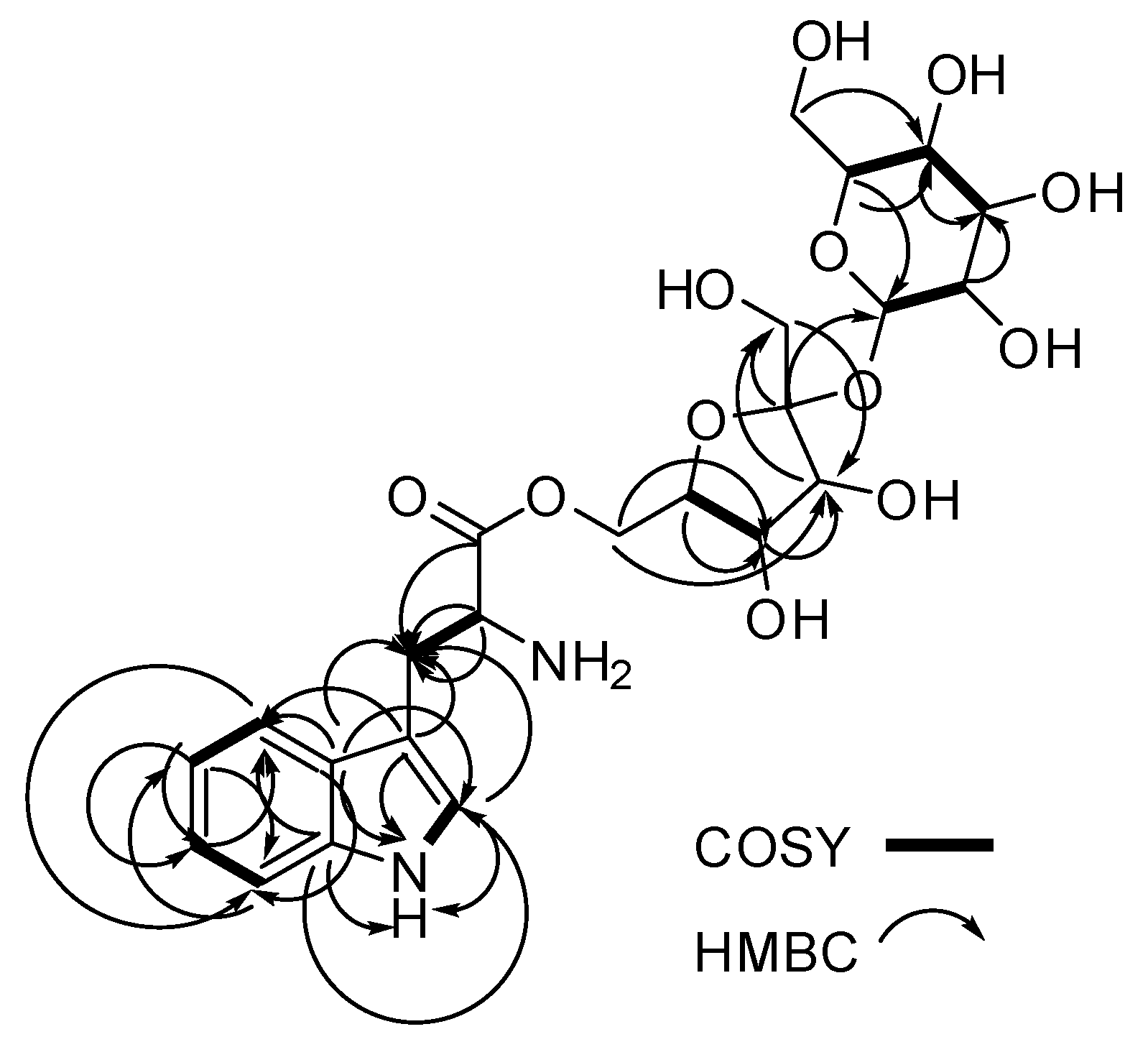
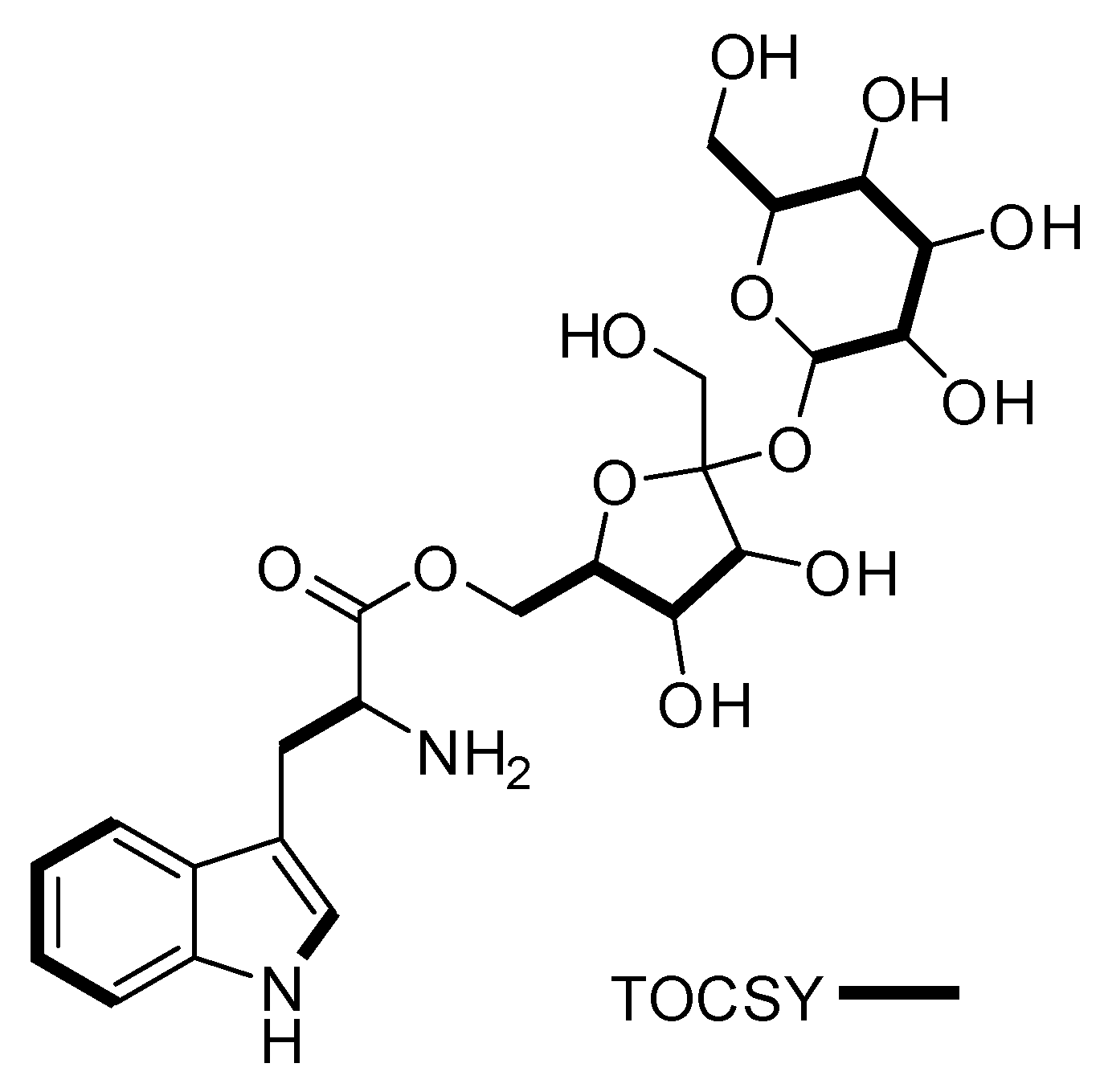
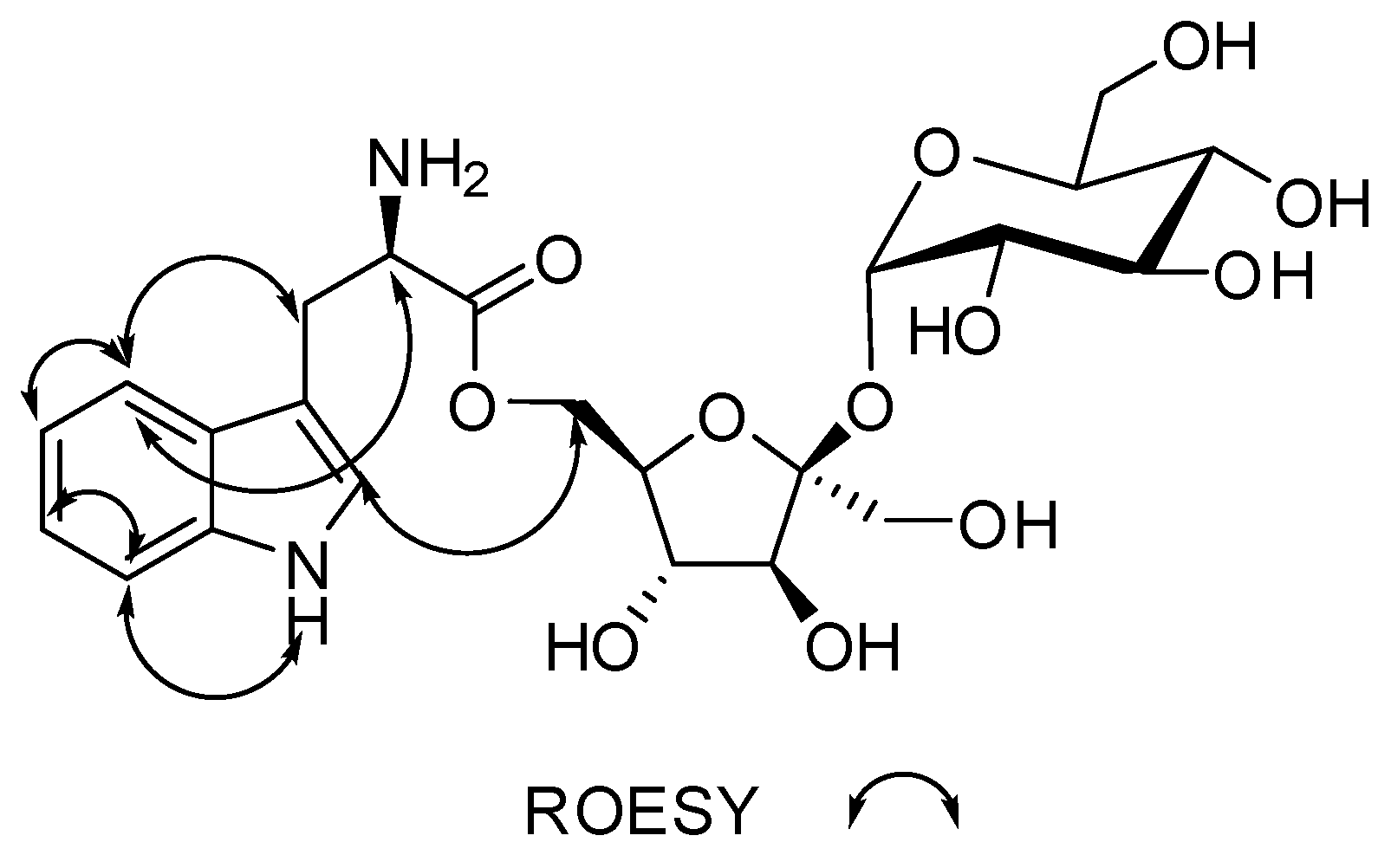
| # | δc mult | δH mult (J Hz) | 1H-1H COSY | HMBC | TOCSY |
|---|---|---|---|---|---|
| 1 NH | 10.9, d (2.9) | 2 | C-2, C-3, C-3a, C-7a | ||
| 2 | 124.2, CH | 7.22, d (2.9) | 1NH | C-3, C-3a, C-7a | 1NH |
| 3 | 109.1, C | ||||
| 3a | 127.2, C | ||||
| 4 | 118.3, CH | 7.59, d (8.1) | 5 | C-3, C-3a, C-6, C-7a | 5, 6,7 |
| 5 | 118.4, CH | 6.97, m | 4 | C-3a, C-7 | 4, 6, 7 |
| 6 | 120.9, CH | 7.06, m | 7 | C-4, C-5, C-7a | 4, 5, 7 |
| 7 | 111.4, CH | 7.35, m | 6 | C-3a, C-4, C-5 | 4, 5, 6 |
| 7a | 136.3, C | ||||
| 8 | 26.9, CH2 | 3.02, dd (15.3, 8.7) 3.32, dd (15.1, 4.4) | 8, 9 8, 9 | C-2, C-3, C-3a, C-9, C-10 | 9, 2 |
| 9 | 54.5, CH | 3.55, m | 8 | 8 | |
| 9 NH2 | 3.59, dd (2.3, 4.3) | ||||
| 10 | 170.8, C | ||||
| 1′ | 62.2, CH2 | 3.41, d (3.1) | C-2′, C-3′ | 3′, 4′, 6′ | |
| 2′ | 104.0, C | ||||
| 3′ | 77.2, CH | 3.89, d (8.1) | C-1′, C-4′, C-6′ | 4′, 5′ | |
| 4′ | 74.3, CH | 3.79, t (7.7) | 5′ | C-1′, C-5′, C-6′ | 3′, 5′ |
| 5′ | 82.5, CH | 3.57, dt (7.7, 2.2) | 4′ | 3′, 4′ | |
| 6′ | 62.1, CH2 | 3.41, d (2.3) | C-10 | 1′, 3′, 4′ | |
| 1′′ | 91.8, CH | 5.19, d (4.1) | 2′′ | C-5′′, C-2′ | 5′′, 2′′, |
| 2′′ | 71.7, CH | 3.20, dd (9.7, 3.9) | 1′′ | 5′′, 3′′, 4′′, 1′′ | |
| 3′′ | 72.8, CH | 3.49, t (9.7) | 4′′ | C-2′′, C-4′′ | 5′′, 2′′, 4′′, 1′′ |
| 4′′ | 69.9, CH | 3.14, t (9.5) | 3′′, 5′′ | C-5′′, C-6′′ | 5′′, 6′′, 2′′, 1′′ |
| 5′′ | 72.9, CH | 3.65, ddd (9.7, 4.7, 2.4) | 4′′ | 1′′, 2′′, 3′′, 4′′ | |
| 6′′ | 60.5, CH2 | 3.52, dd (10, 4.8,) 3.50, dd (10, 2.6) | 5′′, 4′′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyeremeh, K.; Kwain, S.; Tetevi, G.M.; Camas, A.S.; Camas, M.; Dofuor, A.K.; Deng, H.; Jaspars, M. α-d-Glucopyranosyl-(1→2)-[6-O-(l-tryptophanyl)-β-d-fructofuranoside]. Molbank 2019, 2019, M1066. https://doi.org/10.3390/M1066
Kyeremeh K, Kwain S, Tetevi GM, Camas AS, Camas M, Dofuor AK, Deng H, Jaspars M. α-d-Glucopyranosyl-(1→2)-[6-O-(l-tryptophanyl)-β-d-fructofuranoside]. Molbank. 2019; 2019(2):M1066. https://doi.org/10.3390/M1066
Chicago/Turabian StyleKyeremeh, Kwaku, Samuel Kwain, Gilbert Mawuli Tetevi, Anil Sazak Camas, Mustafa Camas, Aboagye Kwarteng Dofuor, Hai Deng, and Marcel Jaspars. 2019. "α-d-Glucopyranosyl-(1→2)-[6-O-(l-tryptophanyl)-β-d-fructofuranoside]" Molbank 2019, no. 2: M1066. https://doi.org/10.3390/M1066
APA StyleKyeremeh, K., Kwain, S., Tetevi, G. M., Camas, A. S., Camas, M., Dofuor, A. K., Deng, H., & Jaspars, M. (2019). α-d-Glucopyranosyl-(1→2)-[6-O-(l-tryptophanyl)-β-d-fructofuranoside]. Molbank, 2019(2), M1066. https://doi.org/10.3390/M1066







