1. Introduction
Nitrogen–sulfur containing heterocyclic ligands, of which the title 1,2,4-triazole-5-thione is an example, are well known for their pharmacological potential [
1,
2] and ability to coordinate metals [
3]. This interest extends to the title compound, 4-(4-chlorophenyl)-4,5-dihydro-1
H-1,2,4-triazole-5-thione (
1), first reported in 1959 [
4,
5], which has been investigated recently for anti-bacterial activity but found to be inactive against the studied Gram-positive and Gram-negative bacteria [
6]. Furthermore, silver complexes of
1 have been patented, most recently for applications as photothermographic materials [
7]. Herein the synthesis, spectroscopic characterization, and X-ray crystal structure determination of the title compound,
1,
Scheme 1, are described.
2. Results and Discussion
The title compound (
1) was prepared in good yield (73%) from the hetero-cyclization reaction of 4-chlorophenyl isothiocyanate and formic hydrazide, following a literature procedure [
6]. The final product was characterized by spectroscopy (
1H and
13C{
1H} NMR, IR, and UV); see
Supplementary Materials for original spectra. The NMR data showed the expected resonances and integration (
1H). The most notable feature in the
13C{
1H} NMR spectrum is the downfield resonance, at
δ 167.9 ppm, ascribed to the quaternary-C1 atom. The absence of a resonance due to S-H suggests the presence of the thione tautomer in CDCl
3 solution. In the IR spectrum, characteristic bands ascribed to
ν(N-H) [3139 (m)],
ν(C-N) [1460 (vs)], and
ν(C=S) [1290 (vs)] were observed. In the UV spectrum, there are two prominent bands observed at
λabs 221 and 277 nm, which are attributed to
π‒
π* and
n‒
π* transitions, respectively. In the absence of definitive structural data, an X-ray crystallographic investigation of
1 was also undertaken.
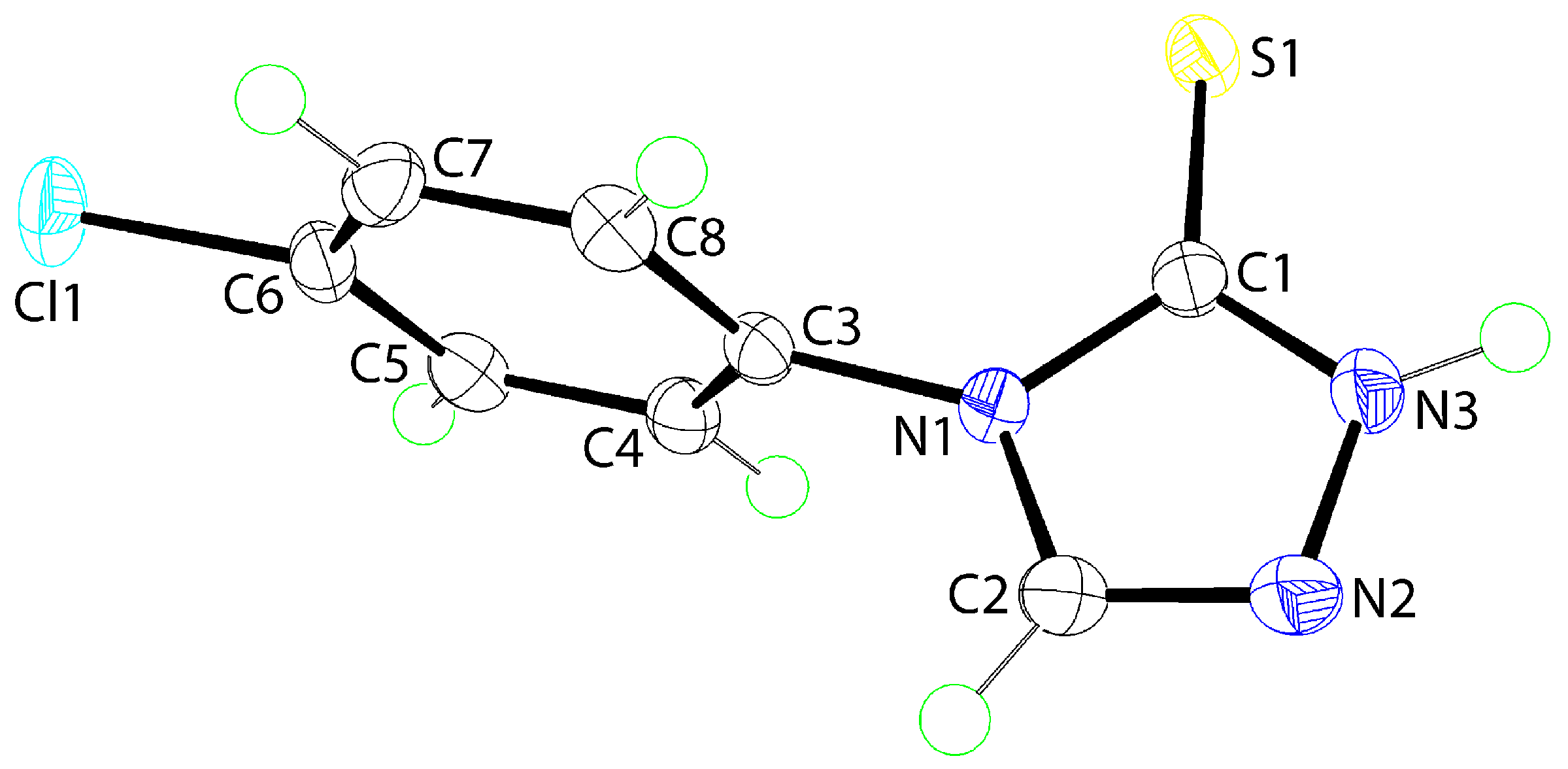
The molecular structure of
1 is shown in
Figure 1. The five-membered ring is strictly planar as seen in the r.m.s. deviation of the five fitted atoms of 0.0056 Å. The appended S1 and C3 atoms lie to opposite sides of the plane by 0.062(2) and 0.149(2) Å, respectively. This planarity in this part of the structure is consistent with significant delocalization of
π-electron density over the ring, as seen in the bond lengths collated in the figure caption. The dihedral angle between the five- and six-membered rings of 82.70(5)° indicates a near to orthogonal relationship. The molecule exists as the thione tautomer, as opposed to the thiol form, as evidenced by the C1=S1 bond length of 1.6840(14) Å. Several authors have suggested
1 to exist as the thiol tautomer [
4,
5] and this might account for some of the discrepancies in the reported melting points (see
Section 3.2). The present assignment of the thione/thioamide form is fully in accord with expectation based on recent computational chemistry investigations of related systems [
8]. As expected, the external angles subtended at the quaternary-C1 atom by the thione-S1 atom are significantly wider than the internal angle. This is equally true for the angles subtended at the tertiary amine-N1 atom. Finally, the angle subtended at the amine-N3 atom of 113.71(11)° is wider than that subtended at the imine-N2 atom of 103.11(11)°, an observation entirely consistent with protonation at the former atom.
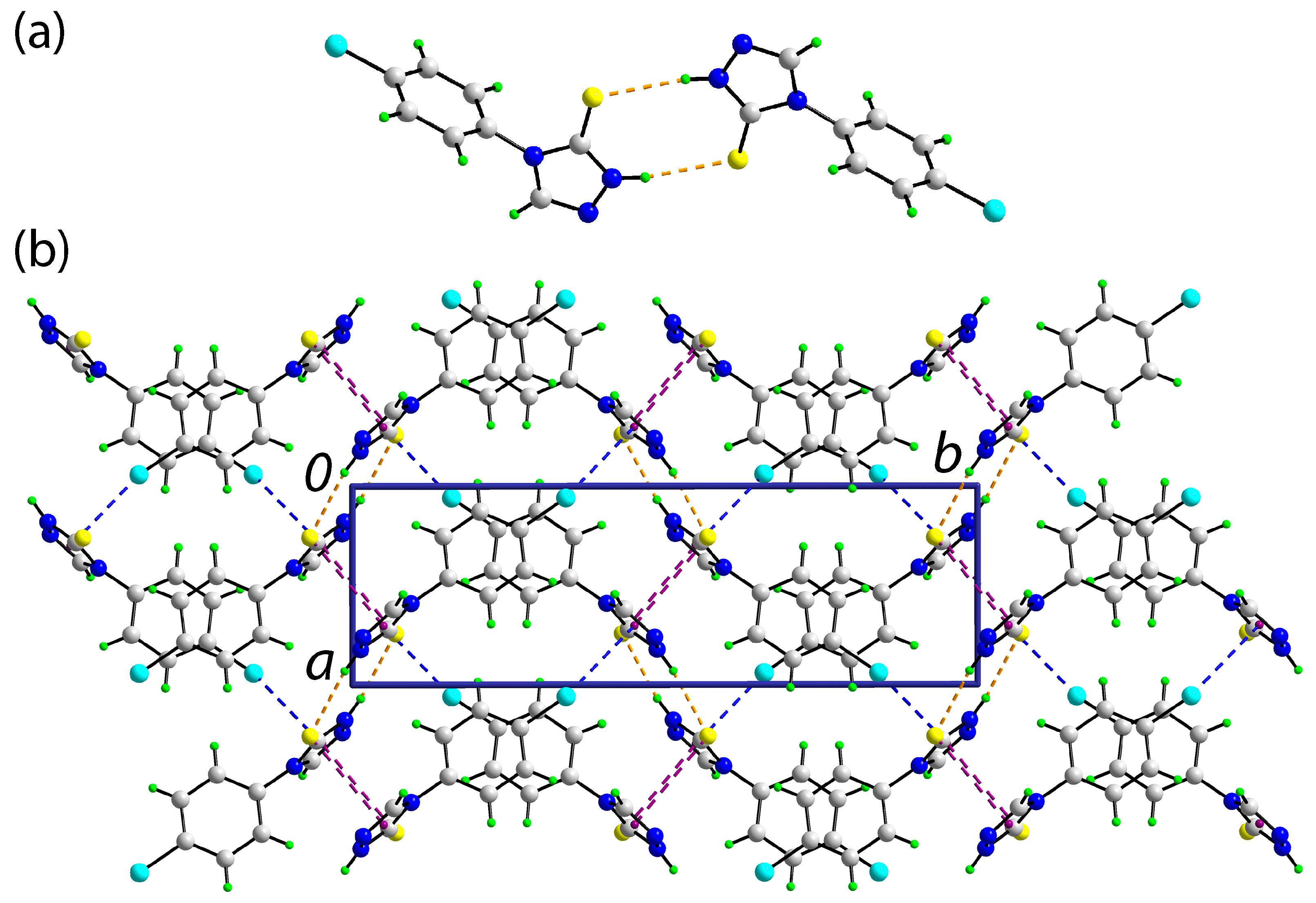
The molecular packing in the crystal of
1 features thioamide-N1–H⋯S1(thione) hydrogen bonds, which lead to a supramolecular dimer via an eight-membered {⋯HNCS}
2 synthon, disposed about a 2-fold axis of symmetry,
Figure 2a; geometric details characterizing intermolecular interactions discussed herein are given in the figure caption. The dimers are connected into a three-dimensional architecture,
Figure 2b, by side-on (parallel) C=S⋯
π(triazolyl) and end-on C-Cl⋯
π(triazolyl) interactions pointing to the pivotal role of the triazolyl ring in the supramolecular association in
1.
There are two closely related literature precedents that may be compared with
1. The first of these has a N1-bound methyl group rather than the 4-chlorophenyl substituent of
1 [
9]. Here, two molecules comprise the crystallographic asymmetric unit and the key C=S [1.675(3) and 1.675(3) Å] and C=N [1.301(4) and 1.301(3) Å] bond lengths match those in
1. However, the supramolecular association is quite distinctive in that amine-N-H⋯N(imine) hydrogen bonds form to connect four molecules into a ring sustained by a 12-membered {⋯HNN}
4 synthon with 2-fold symmetry. The second structure has a 2-morpholinoethyl substituent at the ring-N1 atom [
10]. The C=S [1.674(3) Å] and C=N [1.296(2) Å] bond lengths match those cited above. In the molecular packing, yet another supramolecular motif is noted in that zigzag (propagated by glide symmetry) supramolecular chains are formed via amine-N-H⋯N(morpholine) hydrogen bonding.
In conclusion, X-ray crystallography on 1 reveals the molecule exists as the thione tautomer and shows an almost orthogonal relationship between the constituent aromatic rings. The most prominent feature of the molecular packing is the formation of supramolecular dimers via thioamide-N–H⋯S(thione) hydrogen bonds.
3. Materials and Methods
3.1. General Information
All standard chemicals and solvents were sourced from Merck (Darmstadt, Germany) and used without further purification. The melting point was determined on a Biobase automatic melting point apparatus MP450 (Biobase Group, Jinan, Shandong Province, China). The IR spectrum was measured on a Bruker Vertex 70v FTIR (Billerica, MA, USA) spectrophotometer from 4000 to 80 cm−1. Elemental analyses were performed on a Leco TruSpec Micro CHN Elemental Analyzer (Saint Joseph, MI, USA). 1H and 13C{1H} NMR spectra were recorded in CDCl3 solution on a Bruker Ascend 400 MHz NMR (Billerica, MA, USA) spectrometer with chemical shifts relative to tetramethylsilane. The optical absorption spectra were obtained from an acetonitrile solution of 1 × 10−5 M in the range 200–800 nm on a Shimadzu UV-3600 plus UV/VIS/NIR (Shimadzu Corporation, Kyoto, Japan) spectrophotometer.
3.2. Synthesis and Characterization of 1
Formic hydrazide (0.01 mol, 0.60 g) was dissolved in acetone (5 mL) and followed by the addition of 4-chlorophenyl isothiocyanate (0.01 mol, 1.70 g) in acetone (15 mL). The resulting mixture was stirred for 2 h and then left for slow evaporation under ambient conditions. Colorless crystals were collected after ten days. Yield: 1.55 g, 73%. M. pt: 213.0–214.5 °C cf. 214 °C [
4], 183–186 [
5] and 240 °C [
6]. Anal. Calc. for C
8H
6ClN
3S: C, 45.39; H, 2.86; N, 19.85. Found: C, 45.11; H, 3.02; N, 19.67. IR (cm
−1): 3139 (m)
ν(N-H), 1460 (vs)
ν(C-N), 1290 (vs)
ν(C=S).
1H NMR (CDCl
3):
δ 11.28 (s, br, 1H, NH), 7.91 (s, 1H, CH), 7.57–7.52 (m, br, 4H, aryl-H).
13C{
1H} NMR (CDCl
3):
δ 167.9 (Cq), 140.5 (NCN), 135.7 (Ph, C1), 132.2 (Ph, C4), 129.9 (Ph, C3), 126.9 (Ph, C2). UV (acetonitrile; nm, L·cm
−1·mol
−1):
λabs = 277, ε = 8,100;
λabs = 221, ε = 20,800.
3.3. Crystallography
Intensity data for
1 were measured at T = 100(2) K on a XtaLAB Synergy Dual AtlasS2 (Rigaku Polska SP. Z O O, Wrocław, Poland) diffractometer fitted with Cu Kα radiation (
λ = 1.54184 Å) using
ω-scans so that
θmax was 67.0°. Data reduction, including absorption correction, was accomplished with CrysAlis Pro [
11]. Of the 10,734 reflections measured, 1,564 were unique (
Rint = 0.024), and of these, 1,522 data satisfied the
I ≥ 2
σ(
I) criterion of observability. The structure was solved by direct methods [
12] and refined (anisotropic displacement parameters and C-bound H atoms in the riding model approximation) on
F2 [
13]. The thioamide-H atom was located from a difference map and refined with a N-H restraint of 0.88 ± 0.01 Å. A weighting scheme of the form
w = 1/[
σ2(
Fo2) + (0.033
P)
2 + 0.400
P] was introduced, where
P = (
Fo2 + 2
Fc2)/3). Based on the refinement of 148 parameters, the final values of
R and
wR (all data) were 0.023 and 0.062, respectively. The molecular structure diagram was generated with ORTEP for Windows [
14] and the packing diagram using DIAMOND [
15].
Crystal data for C8H6ClN3S (1): M = 211.67, monoclinic, P21/c, a = 6.23170(10), b = 19.4611(2), c = 7.25960(10) Å, β = 95.9860(10)°, V = 875.61(2) Å3, Z = 4, Dx = 1.606 g cm−3, F(000) = 432 and μ = 5.688 mm−1. CCDC deposition number: 1891647.