N-[4-(1-Methyl-1H-imidazol-2-yl)-2,4′-bipyridin-2′-yl]benzene-1,4-diamine
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
Synthesis of N-[4-(1-Methyl-1H-imidazol-2-yl)-2,4′-bipyridin-2′-yl]benzene-1,4-diamine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forte, B.; Malgesini, B.; Piutti, C.; Quartieri, F.; Scolaro, A.; Papeo, G. A submarine journey: The pyrroleimidazole alkaloids. Mar. Drugs 2009, 7, 705–753. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Zhou, C.H.; Luo, K.; Chen, H.; Lan, J.B.; Xie, R.G. Chiral imidazole metalloenzyme models: Synthesis and enantioselective hydrolysis for α-amino acid esters. J. Mol. Catal. A Chem. 2006, 260, 288–294. [Google Scholar] [CrossRef]
- Jin, Z. Muscarine, imidazole, oxazole, and thiazole alkaloids. Nat. Prod. Rep. 2011, 28, 1143–1191. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Sharma, A.; Gupta, G.K.; Singh, R. Imidazoles as potential antifungal agents: A review. Mini Rev. Med. Chem. 2013, 13, 1626–1655. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Foroumadi, A.; Falahati, M.; Lotfali, E.; Rajabalian, S.; Ebrahimi, S.; Farahyar, S.; Shafiee, A. 2-Hydroxyphenacyl azoles and related azolium derivatives as antifungal agents. Bioorg. Med. Chem. Lett. 2007, 18, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Tiwari, V.K.; Verma, S.S.; Chaturvedi, V.; Bhatnagar, S.; Sinha, S.; Gaikwad, A.N.; Tripathi, R.P. Synthesis and antitubercular screening of imidazole derivatives. Eur. J. Med. Chem. 2009, 44, 3350–3355. [Google Scholar] [CrossRef] [PubMed]
- Isloor, A.M.; Kalluraya, B.; Shetty, P. Regioselective reaction: synthesis, characterization and pharmacological studies of some new Mannich bases derived from 1,2,4-triazoles. Eur. J. Med. Chem. 2009, 44, 3784–3787. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A.; Takriff, M.S. Antimicrobial and antioxidant activities of new metal complexes derived from 3-aminocoumarin. Molecules 2011, 16, 6969–6984. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.H.; Mohamad, A.B. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules 2011, 16, 6833–6843. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, A.A.H.; Al-Amiery, A.A.; Musa, A.Y.; Mohamad, A.B. The Antioxidant Activity of New Coumarin Derivatives. Int. J. Mol. Sci. 2011, 12, 5747–5761. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.A. Antifungal Activities of New Coumarins. Molecules 2012, 17, 5713–5723. [Google Scholar] [CrossRef] [PubMed]
- Al-Majedy, Y.K.; Al-Duhaidahawi, D.L.; Al-Azawi, K.F.; Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B. Coumarins as Potential Antioxidant Agents Complemented with Suggested Mechanisms and Approved by Molecular Modeling Studies. Molecules 2016, 21, 135. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.H.; Mohamad, A.B. New Coumarin Derivative as an Eco-Friendly Inhibitor of Corrosion of Mild Steel in Acid Medium. Molecules 2015, 20, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Al-Majedy, Y.K.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.B. Synthesis and Characterization of Some New 4-Hydroxy-coumarin Derivatives. Molecules 2014, 19, 11791–11799. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, A.A.H.; Mohamad, A.B.; Hammed, L.A.; Al-Amiery, A.A.; San, N.H.; Musa, A.Y. Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by New Coumarin. Materials 2014, 7, 4335–4348. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Kadihum, A.; Mohamad, A.B.; How, C.K.; Junaedi, S. Inhibition of Mild Steel Corrosion in Sulfuric Acid Solution by New Schiff Base. Materials 2014, 7, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Alobaidy, A.H.M.; Mohamad, A.B.; Hoon, P.S. Novel Corrosion Inhibitor for Mild Steel in HCl. Materials 2014, 7, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B.; Musa, A.Y.; Li, C.J. Electrochemical Study on Newly Synthesized Chlorocurcumin as an Inhibitor for Mild Steel Corrosion in Hydrochloric Acid. Materials 2013, 6, 5466–5477. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B.; Junaedi, S. A Novel Hydrazinecarbothioamide as a Potential Corrosion Inhibitor for Mild Steel in HCl. Materials 2013, 6, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
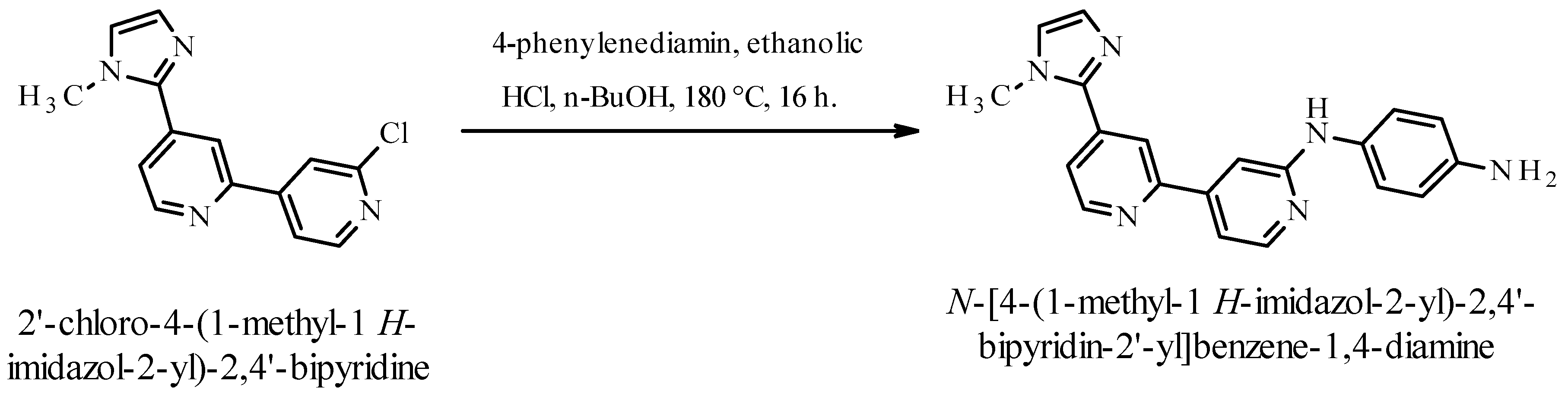
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinad, D.S.; AL-Duhaidahaw, D.L.; Al-Amiery, A.; Kadhum, A.A.H. N-[4-(1-Methyl-1H-imidazol-2-yl)-2,4′-bipyridin-2′-yl]benzene-1,4-diamine. Molbank 2018, 2018, M1030. https://doi.org/10.3390/M1030
Zinad DS, AL-Duhaidahaw DL, Al-Amiery A, Kadhum AAH. N-[4-(1-Methyl-1H-imidazol-2-yl)-2,4′-bipyridin-2′-yl]benzene-1,4-diamine. Molbank. 2018; 2018(4):M1030. https://doi.org/10.3390/M1030
Chicago/Turabian StyleZinad, Dhafer S., Dunya L. AL-Duhaidahaw, Ahmed Al-Amiery, and Abdul Amir H. Kadhum. 2018. "N-[4-(1-Methyl-1H-imidazol-2-yl)-2,4′-bipyridin-2′-yl]benzene-1,4-diamine" Molbank 2018, no. 4: M1030. https://doi.org/10.3390/M1030
APA StyleZinad, D. S., AL-Duhaidahaw, D. L., Al-Amiery, A., & Kadhum, A. A. H. (2018). N-[4-(1-Methyl-1H-imidazol-2-yl)-2,4′-bipyridin-2′-yl]benzene-1,4-diamine. Molbank, 2018(4), M1030. https://doi.org/10.3390/M1030





