2,3,4-Trioxo-1-(1H-pyrrolo[2,3-b]pyridin-7-ium-7yl)-cyclobutan-1-ide
Abstract
:1. Introduction
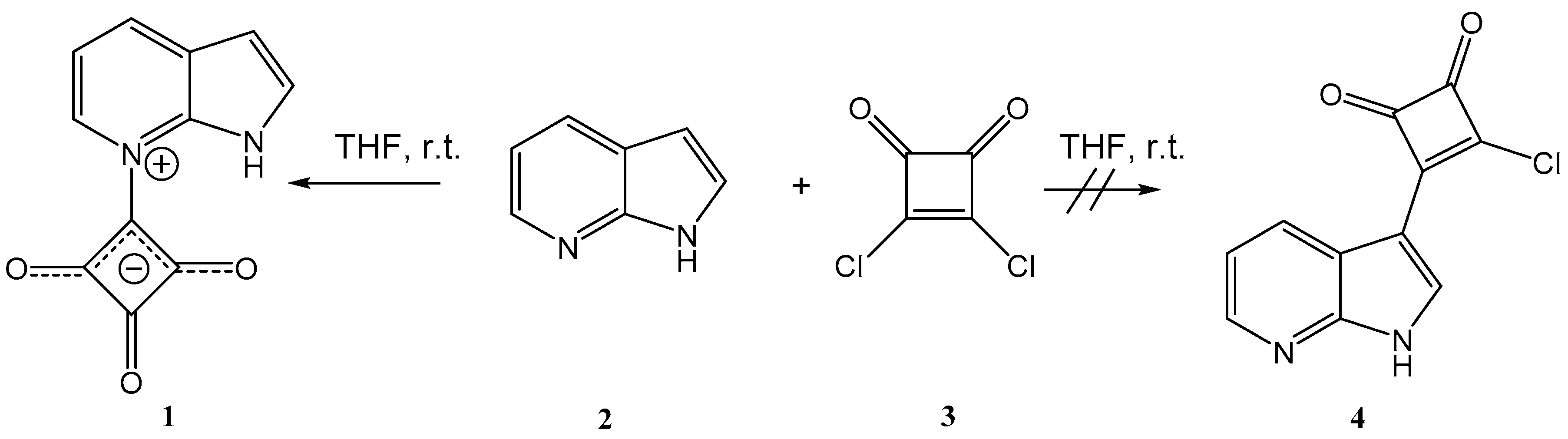
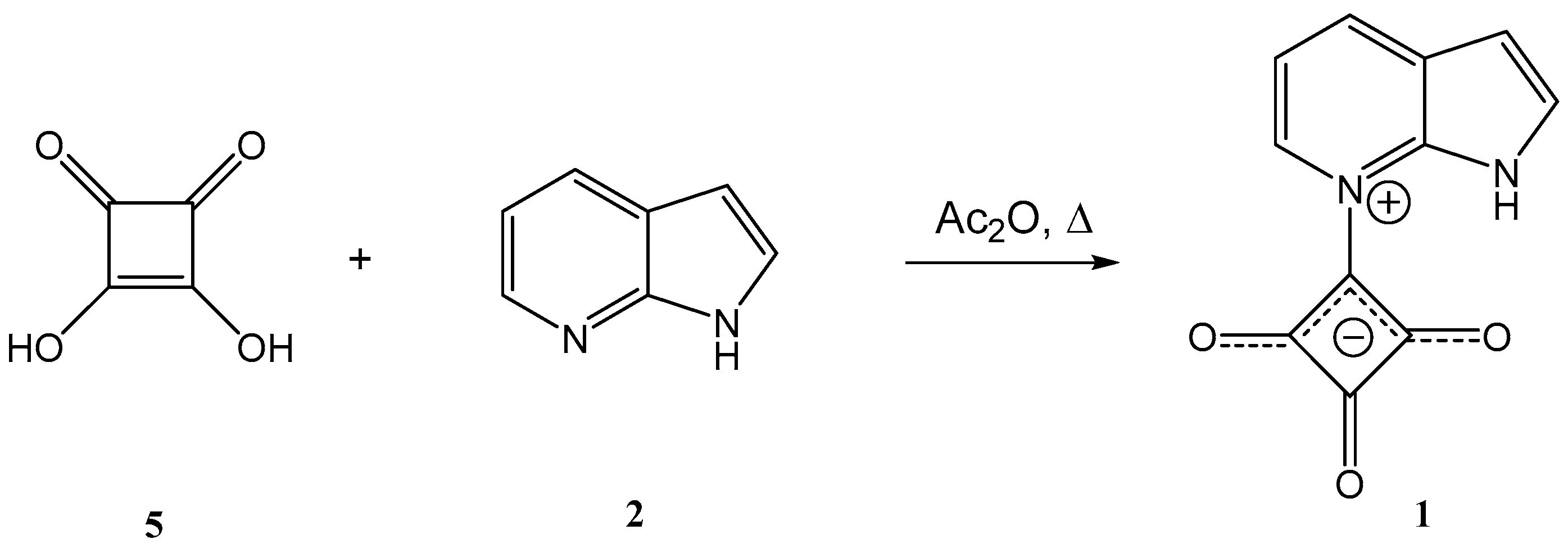
2. Results and Discussion
3. Materials and Methods
3.1. Instrumentation
3.2. Syntheses
3.2.1. Synthesis of 2,3,4-trioxo-1-(1H-pyrrolo[2,3-b]pyridin-7-ium-7-yl)-cyclobutan-1-ide (1); Method 1
3.2.2. Synthesis of 2,3,4-trioxo-1-(1H-pyrrolo[2,3-b]pyridin-7-ium-7-yl)cyclobutan-1-ide (1); Method 2
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Janetka, J.W.; Ashwell, S. Checkpoint kinase inhibitors: A review of the patent literature. Expert Opin. Ther. Pat. 2009, 19, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Wang, Y.M.; Han, Y.X.; Liu, L.; Jin, J.; Yi, H.; Li, Z.R.; Jiang, J.D.; Boykin, D.W. Synthesis and antitumor activity of novel 3,4-diaryl squaric acid analogs. Eur. J. Med. Chem. 2013, 65, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. Bifunctional amine-squaramides: Powerful hydrogen-bonding organocatalysts for asymmetric domino/cascade reactions. Adv. Synth. Catal. 2015, 357, 253–281. [Google Scholar] [CrossRef]
- Kunick, C.; Lande, H.; Gruenefeld, J.; Dzikowski, R.; Nasereddin, A. New Indole Compounds Having Antiprotozoal Activity and Its Use as Well as Methods for Producing the Same. Patent WO/2017/008826, 19 January 2017. [Google Scholar]
- Schmidt, A.H.; Thiel, S.H.; Gaschler, O. Oxocarbons and related compounds. Part 24. Chlorosquarylation of indoles. J. Chem. Soc. Perkin Trans. 1 1996, 6, 495–496. [Google Scholar] [CrossRef]
- Lande, D.H.; Messal, M.; Reimann, V.; Renger, C.; Rurka, C.; Schoemaker, J.; Schröder, N.K.; Grünefeld, J. 3-Chloro-4-(5-methoxy-1H-indol-3-yl)cyclobut-3-ene-1,2-dione. Molbank 2013, 2013, M805. [Google Scholar] [CrossRef]
- Grünefeld, J.; Zinner, G. Neue Oxokohlenstoff-Betaine der Quadratsäure. Chem.-Ztg. 1984, 108, 112. [Google Scholar]
- Schmidt, A.H.; Aimène, A.; Schneider, M. Eine neue Klasse von Stickstoff-Betainen der Quadratsäure. Synthesis 1984, 436–439. [Google Scholar] [CrossRef]
- Schmidt, A.H.; Becker, U.; Aimène, A. Ein neues, einfaches Verfahren zur Darstellung von Ammonium- und Phosphonium-Betainen der Quadratsäure. Tetrahedron Lett. 1984, 25, 4475–4478. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lande, D.H.; Kunick, C.; Grünefeld, J. 2,3,4-Trioxo-1-(1H-pyrrolo[2,3-b]pyridin-7-ium-7yl)-cyclobutan-1-ide. Molbank 2018, 2018, M1026. https://doi.org/10.3390/M1026
Lande DH, Kunick C, Grünefeld J. 2,3,4-Trioxo-1-(1H-pyrrolo[2,3-b]pyridin-7-ium-7yl)-cyclobutan-1-ide. Molbank. 2018; 2018(4):M1026. https://doi.org/10.3390/M1026
Chicago/Turabian StyleLande, Duc Hoàng, Conrad Kunick, and Johann Grünefeld. 2018. "2,3,4-Trioxo-1-(1H-pyrrolo[2,3-b]pyridin-7-ium-7yl)-cyclobutan-1-ide" Molbank 2018, no. 4: M1026. https://doi.org/10.3390/M1026
APA StyleLande, D. H., Kunick, C., & Grünefeld, J. (2018). 2,3,4-Trioxo-1-(1H-pyrrolo[2,3-b]pyridin-7-ium-7yl)-cyclobutan-1-ide. Molbank, 2018(4), M1026. https://doi.org/10.3390/M1026




