Abstract
In this paper, an accessible chiral pool synthesis of benzyl (R)-2-(acetylthio)propanoate (acetylthiolactate), which is less odorous than the methyl or ethyl analogue, was performed through a clean SN2 displacement reaction using available AcSK with tris[2-(2-methoxyethoxy)]ethylamine (TDA-1), starting from commercially available benzyl (S)-lactate in 76%, 94% ee (2 steps). Deprotection of the acetyl group using N,N-dimethylethylenediamine afforded benzyl (R)-2-sulfanylpropanoate in 93% yield with 90% ee. These two sulfur-containing benzyl esters were sufficiently odorless to be purified by column chromatography. Direct HPLC analysis was applied to determine the enantiomeric excess without thiazolidin-4-one derivatizations. A complementary debenzylation of benzyl (R)-2-(acetylthio)propanoate was also performed using HBr/AcOH to afford (R)-2-(acetylthio)propanoic acid without critical racemization in 92% yield with 92% ee.
1. Introduction
Synthetic chemistry of optically pure secondary thiols as the isoster of the corresponding chiral alcohols has attracted much attention [1,2]. Chiral 2-sulfanyl (classically, α-mercapto) carboxylic acids, and esters are well-recognized synthetic building blocks for distinctive derivatives of chiral 2-hydroxycarboxylic acids and esters in natural products and pharmaceutical syntheses. Among them, chiral 2-sulfanylpropanoic acid and esters 1 (thiolactic acid and esters) serve as the most fundamental chiral synthons (Figure 1). In this paper, we report a straightforward and accessible synthesis of novel benzyl (2R)-2-(acetylthio)propanoate [(R)-2] starting from inexpensive and commercially available benzyl (S)-lactate through mesylation and a clean SN2 displacement reaction using available AcSK with tris[2-(2-methoxyethoxy)]ethylamine (TDA-1) [3]. In addition, convenient direct HPLC analysis was performed to determine the accurate optical purities of (R)-2 and their analogues.
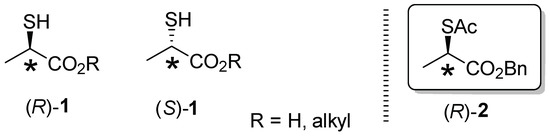
Figure 1.
Chiral thiolactic acid and esters (R)-1, (S)-1, and benzyl (2R)-2-(acetylthio)propanoate (R)-2.
Representative natural products and biologically active agents/compounds installing the chiral thiol segments (R)- and (S)-1 are listed in our recent report along with the display of their structures [3]. Tiopronin [4,5] and thiolactomycin [6,7,8,9] are leading natural antibiotic compounds (Figure 2). Other biologically active agents/compounds are described in chronologic order of their development: antiplatelet activating factor (anti-PAF) antagonists [10,11]; IMP-1, a metallo-β-lactamase inhibitor [12]; vasopeptidase inhibitors [13]; methionine aminopeptidases (MetAPs) active site probes [14]; specific substrates for Streptomyces R61 d,d-peptidases [15]; and a nonsteroidal farnesoid X receptor (FXR) agonist [16].
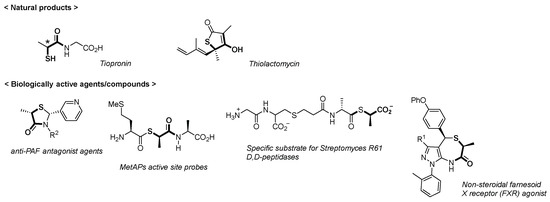
Figure 2.
Representative natural products containing chiral thiol segments (R)- or (S)-1.
Chiral acid and esters 1 have two characteristic synthetic utilities. One is the chiral template methodology [17] using 1,3-oxathiolan-4-ones derived from 1, which involves distinct self-regeneration of the stereocenter [18]. This protocol was successfully applied for the asymmetric synthesis of (5R)-thiolactomycin and its analogues [9,19,20]. Another is a thiazolidin-4-one type chiral ligand derived from 1, which was utilized for a Cu(I)-catalyzed asymmetric conjugate addition to enones, developed by Feringa’s group [20].
Due to the demand, several synthetic methods to access 1 have been developed to date. Scheme 1 shows the most traditional synthesis of (R)-1a starting from chiral alanine, developed by Owen’s [21] and Kellogg’s groups [22]; stereoretentive diazotization-chlorination; SN2 displacement with AcSCs (generated in situ from AcSH and Cs2CO3); and deacetylation sequences. The addressed yields are referred from Townsend and co-workers’ total synthesis of (R)-thiolactomycin [9]. However, this reliable method requires a somewhat tedious step for in situ generation of odorous and hygroscopic AcSCs from AcSH and Cs2CO3, and results in moderate overall yield.
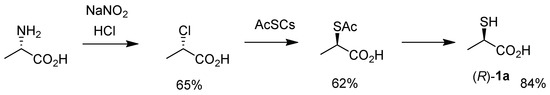
Scheme 1.
Traditional synthesis of chiral thiolactic acid (R)-1a.
Another notable synthesis is copper-catalyzed enantioselective carbenoid insertion to α-diazopropanoate; this method afforded the desired benzyl ester 5 with 77% ee using a chiral bisoxazoline ligand [23]. In connection with our continuing studies on process chemistry and biologically active sulfur- and nitrogen-containing heterocyclic compounds [24,25,26,27], we planned to develop a practical and robust synthesis of chiral building blocks 1.
2. Results
Our previous report described the synthesis of 1 as well as the related α-sulfanyl succinate and mandelate and their accurate HPLC optical purity determinations by derivatization under nearly neutral conditions [Ti(Oi-Pr)4/N-benzylidenemethylamine] to thiazolidin-4-ones [3]. However, in the case of 1, this method required the isolation of methyl acetylthiolactate and methyl thiolactate, both of which have a highly unpleasant odor due to their high volatility. To address this problem, we investigated an alternative protocol using benzyl analogues. Scheme 2 outlines the reaction sequence. Cheap and available benzyl (S)-lactate 3 was converted to benzyl (S)-methanesulfonate 4. The key clean SN2 displacement reaction of 4 was conducted using commercially available AcSK—an odorless, less hygroscopic, easy-to handle solid in bench-top procedures—and compared with liquid AcSH and Cs2CO3 [22]. Condition A (TDA-1, tris[2-(2-methoxyethoxy)]ethylamine additive, AcOEt solvent) is slightly superior to conventional condition B (no additive, DMF solvent) with regard to yield, enantiomeric excess, and well-equalized suspension formation during the reaction. TDA-1 is an inexpensive and less toxic cryptand modified for 18-crown-6.
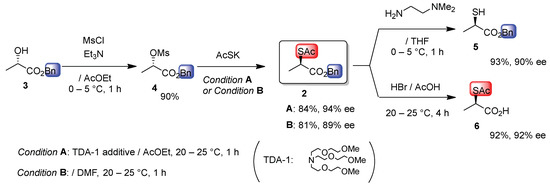
Scheme 2.
Synthesis of benzyl (R)-2-(acetylthio)propanoate (2) and derivatization leading to (R)-benzyl 2-sulfanyl ester 5 and (R)-2-acetylthio acid 6.
Benzyl (R)-acetylthioester 2 was considerably less odorous than the corresponding methyl or ethyl acetylthioester and, therefore, readily purified by column chromatography. Mild deacetylation of 2 with N,N-dimethylethylenediamine afforded benzyl (R)-2-sulfanylpropanoate (5) in 93% yield with 90% ee. Complementary debenzylation of 2 afforded (R)-2-(acetylthio)propanoic acid (6) upon treatment with HBr/AcOH in 92% yield, also without significant racemization (92% ee). Catalytic hydrogenation to remove the benzyl group failed to proceed (no reaction or decomposition) in any of the cases examined (H2, 10% Pd/C; TBS-H, Pd(OAc)2, Et3N; Et3SiH, Pd(OAc)2, Ph3P; PdCl2).
The enantiomeric purity determination of 2 and 5 is a crucial subject. Two methods of determining the optical purity have been reported to date. The seminal method was developed by Kellogg and Feringa’s group utilizing 13C and 31P-NMR determination techniques of phosphonodithiolate derivatives and/or chiral shift reagents [28]. The other more accurate method utilized the neutral derivatization of 2-sulfanylcarboxylic acids and esters to thiazolidin-4-ones, which were subjected to HPLC analyses [3,11]. Notably, direct HPLC analysis of 2 and 5 to determine the enantiomeric excess was performed with the aid of UV detection of the benzyl group; derivatization to the corresponding thiazolidin-4-ones was omitted.
3. Experimental Section
General
All reactions were carried out in oven-dried glassware under an argon atmosphere. Flash column chromatography was performed with silica gel Merck 60 (230–400 mesh ASTM, Tokyo, Japan). TLC analysis was performed on 0.25 mm Silicagel Merck 60 F254 plates (Tokyo, Japan). Melting points were determined on a hot stage microscope apparatus (AS ONE, ATM-01, Tokyo, Japan) and were uncorrected. NMR spectra were recorded on a JEOL DELTA 300 or JEOLRESONANCE ECX-500 spectrometer (Tokyo, Japan), operating at 300 MHz or 500 MHz for 1H-NMR and 75 MHz or 120 MHz for 13C-NMR. Chemical shifts (δ ppm) in CDCl3 were reported downfield from TMS (=0) for 1H-NMR. For 13C-NMR, chemical shifts were reported in the scale relative to CDCl3 (77.00 ppm) as an internal reference. IR Spectra were recorded on a JASCO FT/IR-5300 spectrophotometer (Tokyo, Japan). Mass spectra were measured on a JEOL JMS-T100LC spectrometer (Tokyo, Japan).
Benzyl (S)-2-[(methylsulfonyl)oxy]propanoate (4)
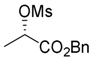
MsCl (7.01 g, 61.2 mmol) was added dropwise to a stirred solution of benzyl (S)-2-hydroxypropanoate (3; 7.35 g, 40.8 mmol) and Et3N (6.19 g, 61.2 mmol) in AcOEt (40 mL) at 0–5 °C for 20 min, and the mixture was stirred at the same temperature for 1 h. Then, N,N-dimethylethylenediamine (2.2 mL) and water (ca. 40 mL) were successively added to the mixture, which was extracted with AcOEt (40 mL × 2). The combined organic phase was washed with water, brine, dried (Na2SO4), and concentrated. The obtained crude oil was purified by SiO2 column chromatography (hexane/AcOEt = 4:1) to give the desired product (9.45 g, 90%). Colorless oil; + 49.2 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ = 1.62 (d, J = 6.9 Hz, 3H), 3.10 (s, 3H), 5.18 (q, J = 6.9 Hz, 1H), 5.20 (d, Jgem = 12.6 Hz, 1H), 5.25 (d, Jgem = 12.6 Hz, 1H), 7.33–7.43 (m, 5H); 13C-NMR (125 MHz, CDCl3): δ = 18.2, 39.0, 67.6, 74.1, 128.2 (2C), 128.6 (3C), 134.7, 169.3; IR (neat): νmax = 1751, 1456, 1352, 1175, 1120, 1086, 1030 cm−1; HRMS (ESI): m/z calcd. for C11H14O5S [M + Na]+ 281.0460; found: 281.0462.
Benzyl (R)-2-(acetylthio)propanoate (2)
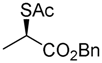
(Condition A) Benzyl (S)-2-[(methylsulfonyl)oxy]propanoate (4; 9.04 g, 35.0 mmol) in AcOEt (5 mL) was added dropwise to a stirred suspension of AcSK (4.40 g, 38.5 mmol) and tris[2-(2-methoxyethoxy)ethyl]amine (TDA-1) (12.5 g, 38.5 mmol) in AcOEt (100 mL) at 20–25 °C for 5 min, and the mixture was vigorously stirred at the same temperature for 1 h to maintain well-equalized suspension formation during the reaction. 1 M HCl aqueous solution (ca. 20 mL) was added to the mixture, which was extracted with AcOEt (40 mL × 2). The combined organic phase was washed with water, brine, dried (Na2SO4), and concentrated. The obtained crude oil was purified by SiO2 column chromatography (hexane/AcOEt = 20:1) to give the desired product (2; 6.98 g, 84%).
Colorless oil; 94% ee, by HPLC analysis (Daicel Chiralcel OD-H column, hexane/2-propanol = 100:1, 1.0 mL/min, 254 nm UV detector), tR = 12.28 min (R) and tR = 11.80 min (S); + 95.9 (c 1.00, CHCl3) 1H-NMR (500 MHz, CDCl3): δ = 1.52 (d, J = 7.5 Hz, 3H), 2.34 (s, 3H), 4.29 (q, J = 7.5 Hz, 1H), 5.17 (s, 2H), 7.31–7.39 (m, 5H); 13C-NMR (125 MHz, CDCl3): δ = 17.5, 30.0, 40.9, 67.2, 127.9 (2C), 128.5 (2C), 135.4, 171.7, 193.7 cm−1; HRMS (ESI): m/z calcd. for C12H14O3S [M + Na]+ 261.0562; found: 261.0559.
(Condition B) In a similar procedure, the use of 4 (1.29 g, 5.0 mmol) and AcSK (0. 69 g, 6.0 mmol) in DMF (10 mL) under the identical conditions gave the desired product (2; 0.95 g, 80%, 89% ee by HPLC analysis).
Benzyl (R)-2-sulfanylpropanoate (5) [23]
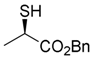
N,N-Dimethylethylenediamine (5.77 mL, 7.12 g, 53.0 mmol) was added dropwise to a stirred solution of benzyl (R)-2-(acetylthio)propanoate (2; 6.31 g, 26.5 mmol) in THF (53 mL) at 0–5 °C for 5 min, and the mixture was stirred at the same temperature for 1 h. 1 M HCl aqueous solution (ca. 20 mL) was added to the mixture, which was extracted with AcOEt (30 mL × 2). The combined organic phase was washed with water, brine, dried (Na2SO4), and concentrated. The obtained crude oil was purified by SiO2 column chromatography (hexane/AcOEt = 20:1) to give the desired product (5; 5.30 g, 93%).
Colorless oil; 90% ee, by HPLC analysis (Daicel Chiralcel OJ-H column, hexane/2-propanol = 150:1, 1.0 mL/min, 254 nm UV detector), tR = 27.20 min (R) and tR = 24.07 min (S); + 15.7 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ = 1.54 (d, J = 6.9 Hz, 3H), 2.17 (d, J = 8.0 Hz, 1H), 3.55 (dq, J = 6.9, 8.0 Hz, 1H), 5.16 (d, Jgem = 12.0 Hz, 1H), 5.19 (d, Jgem = 12.0 Hz, 1H), 7.31–7.41 (m, 5H); 13C-NMR (125 MHz, CDCl3): δ = 21.0, 35.6, 67.0, 128.1 (2C), 128.5 (2C), 135.5, 173.4.
(R)-2-(acetylthio)propanoic acid (6) [29]
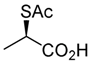
A mixture of benzyl (R)-2-(acetylthio)propanoate (2; 0.48 g, 2.0 mmol) and HBr (ca. 5.1 M in AcOH, 2 mL) was stirred at 20–25 °C for 4 h. Toluene (1 mL) was added to the mixture and was evaporated (azeotropic removal) to give the residue, which was purified by SiO2 column chromatography (hexane/AcOEt = 5:1) to give the desired product (6; 0.27 g, 92%).
Pale yellow oil; + 104.6 (c 1.05, MeOH); [(S)-form; lit. [29] − 114 (c 0.50, MeOH)]. 1H-NMR (500 MHz, CDCl3): δ = 1.53 (d, J = 7.5 Hz, 3H), 2.38 (s, 3H), 4.24 (q, J = 7.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3): δ = 17.1, 30.0, 40.6, 177.6, 194.2.
4. Conclusions
Chiral pool synthesis of novel and less odorous benzyl (R)-2-(acetylthio)propanoate was performed through a clean SN2 displacement reaction using available AcSK with tris[2-(2-methoxyethoxy)]ethylamine (TDA-1) starting from commercially available benzyl (S)-lactate in two steps. Deprotection of the acetyl group using N,N-dimethylethylenediamine afforded benzyl (R)-2-sulfanylpropanoate in good yield without undesirable racemization. These two sulfur-containing benzyl esters were sufficiently odorless to be purified by column chromatography. Benzyl (R)-2-(acetylthio)propanoate (acetylthiolactate) is less odorous than the corresponding methyl or ethyl analogue. Direct HPLC analysis was applied to determine the enantiomeric excess without thiazolidin-4-one derivatizations. Upon treatment with HBr/AcOH, complementary debenzylation of benzyl (R)-2-(acetylthio)propanoate afforded (R)-2-(acetylthio)propanoic acid without significant racemization.
Supplementary Materials
All materials (substrates and reagents) in this work are commercially available with inexpensive price. Copies of the 1H, 13C-NMR spectra for compounds 2, 4, 5, and 6 and copies of HPLC analyses data of 2 and 5 are available in the supplementary information. They and molfiles can be found on line.
Author Contributions
M.K. and S.I. contributed the whole syntheses and assisted literature survey. Y.T. prepared the whole manuscript.
Funding
This research was partially supported by Grant-in-Aids for Scientific Research on Basic Areas (B) “18350056” and (C) 15K05508, Priority Areas (A) “17035087” and “18037068”, and Exploratory Research “17655045” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Conflicts of Interest
The authors declare no conflict of interest. One of the author (Y.T.) offers his warmest congratulations to Ben L. Feringa (University of Groningen, The Netherlands) on being awarded the 2016 Nobel Prize in Chemistry. Dedication to Teruaki Mukaiyama on the celebration of his 90th birthday (Sotuju).
References
- Toru, T.; Bolm, C. Organosulfur Chemistry in Asymmetric Synthesis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Clayden, J.; MacLellan, P. Asymmetric Synthesis of Tertiary Thiols and Thioesters. Beilstein. J. Org. Chem. 2011, 7, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Tanabe, Y. Chiral Syntheses of Methyl (R)-2-Sulfanylcarboxylic Esters and Acids with Optical Purity Determination using HPLC. Chirality 2018, 30, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Labadarios, D.; Davis, M.; Portmann, B.; Williams, R. Paracetamol-induced Hepatic Necrosis in the Mouse: Relationship between Covalent Binding, Hepatic Glutathione Depletion, and Protective Effect of α-Mercaptopropionylglycine. Biochem. Pharmacol. 1977, 26, 31–35. [Google Scholar] [CrossRef]
- Wang, H.; Ma, C.; Zhou, J.; Liu, X.Q. Stereoselective Analysis of Tiopronin Enantiomers in Rat Plasma Using High-Performance Liquid Chromatography-Electrospray Ionization Mass Spectrometry After Chiral Derivatization. Chirality 2009, 21, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Oishi, H.; Noto, T.; Sasaki, M.; Suzuki, K.; Hayashi, T.; Okazaki, H.; Ando, K.; Sawada, M. Thiolactomycin, a New Antibiotic. I. Taxonomy of the Producing Organism, Fermentation and Biological Properties. J. Antibiot. 1982, 35, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, K.; Omura, S. Synthesis and Biological Activities of Thiotetromycin Analogs. J. Antibiot. 1983, 36, 1589–1591. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Salvono, J.M. Total Synthesis of (±) thiolactomycin. Tetrahedron Lett. 1984, 25, 5243–5246. [Google Scholar] [CrossRef]
- McFadden, J.M.; Frehywot, G.L.; Townsend, C.A. A Flexible Route to (5R)-Thiolactomycin, a Naturally Occurring Inhibitor of Fatty Acid Synthesis. Org. Lett. 2002, 4, 3859–3862. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Suzukamo, G.; Komuro, Y.; Imanishi, N.; Morooka, S.; Enomoto, M.; Kojima, A.; Sanemitsu, Y.; Mizutani, M. Structure-Activity Relationship of Optically Active 2-(3-Pyridyl)thiazolidin-4-Ones as an Anti-PAF Antagonist. Tetrahedron Lett. 1991, 32, 379–382. [Google Scholar] [CrossRef]
- Tanabe, Y.; Yamamoto, H.; Murakami, M.; Yanagi, K.; Kubota, Y.; Okumura, H.; Sanemitsu, Y.; Suzukamo, G. Synthetic Study of the Highly Potent and Selective Anti-Platelet Activating Factor Thiazolidin-4-one Agents and Related Compounds. J. Chem. Soc. Perkin Trans. 1 1995, 935–947. [Google Scholar] [CrossRef]
- Greenlee, M.L.; Laub, J.B.; Balkovec, J.M.; Hammond, M.L.; Hammond, G.G.; Pompliano, D.L. Epstein-Toney, J.H. Synthesis and SAR of thioester and Thiol Inhibitors of IMP-1 Metallo-β-Lactamase. Bioorg. Med. Chem. Lett. 1999, 9, 2549–2554. [Google Scholar] [CrossRef]
- Singh, J.; Kronenthal, D.R.; Schwinden, M.; Godfrey, J.D.; Fox, R.; Vawter, E.J.; Zhang, B.; Kissick, T.P.; Patel, B.; Mneimne, O.; et al. Efficient Asymmetric Synthesis of the Vasopeptidase Inhibitor BMS-189921. Org. Lett. 2003, 5, 3155–3158. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.M.; Li, J.Y.; Chen, L.L.; Li, J.; Ye, Q.Z.; Nan, F.J. Design and Synthesis of Chromogenic Thiopeptolide Substrates as MetAPs Active Site Probes. Bioorg. Med. Chem. 2004, 12, 2853–2861. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.; Jolly, R.S. Transpeptidation Reactions of a Specific Substrate Catalyzed by the Streptomyces R61 DD-Peptidase: Characterization of a Chromogenic Substrate and Acyl Acceptor Design. Biochemistry 2005, 44, 9971–9979. [Google Scholar] [CrossRef] [PubMed]
- Marinozzi, M.; Carotti, A.; Sardella, R.; Buonerba, F.; Ianni, F.; Natalini, B.; Passeri, D.; Rizzo, G.; Pellicciari, R. Asymmetric Synthesis of the Four Diastereoisomers of a Novel Non-Steroidal Farnesoid X Receptor (FXR) Agonist: Role of the Chirality on the Biological Activity. Bioorg. Med. Chem. 2013, 21, 3780–3789. [Google Scholar] [CrossRef] [PubMed]
- Strijtveen, B.; Kellogg, R.M. Synthesis and Determination of Enantiomeric Excesses of Non-Racemic Tert-Thiols Derived from Chiral Secondary α-Mercaptocarboxylic Acids. Tetrahedron 1987, 43, 5039–5054. [Google Scholar] [CrossRef]
- Seebach, D.; Neaf, R.; Calderani. α-Alkylation of α-Heterosubstituted Carboxylic Acids without Racemization: EPC-Syntheses of Tertiary Alcohols and Thiols. Tetrahedron 1984, 40, 1313–1324. [Google Scholar] [CrossRef]
- Kapilashrami, K.; Bommineni, G.R.; Machutta, C.A.; Kim, P.; Lai, C.T.; Simmerling, C.; Picart, F.; Tonge, P.J. Thiolactomycin-Based β-Ketoacyl-AcpM Synthase A (KasA) Inhibitors. J. Biol. Chem. 2013, 288, 6045–6052. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A.H.M.; Hof, R.P.; Staal, D.; Kellogg, R.M.; Feringa, B.L. Diastereoselective Synthesis of Pyridyl Substituted Thiazolidin-4-Ones. New Ligands for the Cu(I) Catalyzed Asymmetric Conjugate Addition of Diethylzinc to Enones. Tetrahedron Asymmetry 1997, 8, 539–1543. [Google Scholar] [CrossRef]
- Owen, L.N.; Rahman, M.B. The Synthesis and Reduction of Optically Active 2-Mercaptopropionic Acid and Some Derivatives. J. Chem. Soc. C 1971, 2432–2440. [Google Scholar] [CrossRef]
- Strijtveen, B.; Kellogg, R.M. Synthesis of (Racemization Prone) Optically Active Thiols by SN2 Substitution Using Cesium Thiocarboxylates. J. Org. Chem. 1986, 51, 3664–3671. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhu, S.F.; Cai, Y.; Mao, H.X.; Zhou, Q.L. Copper-Catalyzed Enantioselectivecarbenoidinsertion into S–H Bonds. Chem. Commun. 2009, 5362–5364. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Okabe, T.; Kakimizu, A.; Ohno, N.; Yoshioka, H. An Improved Method for Preparation of N-Alkyl-2(3H)-benzothiazolone. Bull. Chem. Soc. Jpn. 1983, 56, 1255–1256. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sanemitsu, Y. A Covenient Synthesis of 3-Chloromethyl-2(3H)-benzothiazolone. Synthesis 1988, 482–484. [Google Scholar] [CrossRef]
- Sanemitsu, Y.; Kawamura, S.; Tanabe, Y. Regioselective α-Methoxycarbonyl-sulfenylation of Ketones and Aldehydes: A Versatile Method for Preparation of Thiazolones, Thiadiazinones, and 3-Indolethiols. J. Org. Chem. 1992, 57, 1053–1056. [Google Scholar] [CrossRef]
- Shotaro, I.; Nakatsuji, H.; Tanabe, Y. Straightforward Synthesis of N-Methyl-4-(Pin)B-2(3H)-benzothiazol-2-one: A promising Cross-Coupling Reagent. Molbank 2018, 1, M976. [Google Scholar] [CrossRef]
- Strijtveen, B.; Feringa, B.L.; Kellogg, R.M. Methyl Phosphoric Dichloride as Reagent for the Determination of the Enantiomeric Excess of Chiral Thiols. Scope and Limitation. Tetrahedron 1987, 43, 123–130. [Google Scholar] [CrossRef]
- Robl, J.A.; Kronenthal, D.R.; Goderey, J.D., Jr. Bicyclic Carboxylic Acids as Inhibitors of Neutral Endopeptidase and Angiotensin-Converting Enzyme. Euro. Patent EP 629627, 21 December 1994. [Google Scholar]
Sample Availability: Samples of the compounds 2, 5 and 6 are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).