Abstract
A new flavonoid derivative, namely 5,7-dihydroxy-3,6-dimethoxy-3′,4′-methylenedioxyflavone (1), was isolated from the leaves of Melicope glabra (Blume) T.G. Hartley. The structure of 1 was elucidated based on their UV, IR, HRESIMS, and 1D and 2D NMR spectral data.
1. Introduction
Melicope glabra is one species belonging to the Rutaceae family and is found in all of Indonesia Island. The leaves of Melicope glabra are used in Indonesia as traditional medicine for the treatment of fever and cough. According to previous phytochemical studies, the most common secondary metabolites isolated from the genus Melicope are alkaloids [1,2], coumarins [3], acetophenones [4], and flavonoids [5]. Flavonoid derivatives in the genus Melicope have demonstrated their value as a chemical marker. Secondary metabolites from the genus Melicope have shown a wide range of biological and pharmacological applications, owing to such properties as antioxidant [3], antimalarial [1], and anticancer [2]. In the present study, a phytochemical investigation is reported from leaves of Melicope glabra (Blume) T.G. Hartley, focused on the isolation and structural elucidation of a new flavonol derivative, 5,7-dihydroxy-3,6-dimethoxy-3′,4′-methylenedioxyflavone, shown in Figure 1. The cytotoxic activity against murine leukemia P-388 cells and the antioxidant radical scavenging activity toward 2,2-diphenyl-1-picrihydrazyl (DPPH) are also reported.
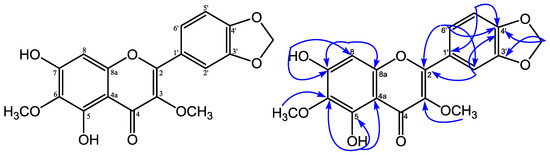
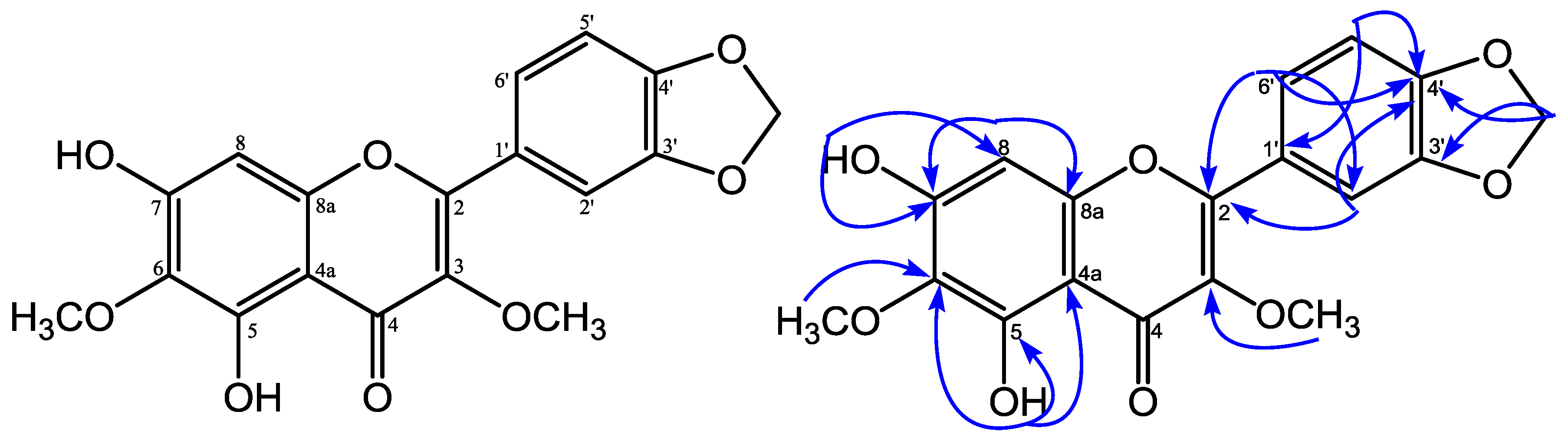
Figure 1.
Structure and selected HMBC correlations for 5,7-dihydroxy-3,6-dimethoxy-3′,4′-methylenedioxyflavone.
2. Results and Discussion
5,7-Dihydroxy-3,6-dimethoxy-3′,4′-methylenedioxyflavone was obtained as a yellow solid and showed an m.p. of 119–121 °C. HRESIMS measurement of 1 revealed a pseudomolecular ion peak [M − H]− at m/z 357.0610 (calcd. 357.0610), consistent with a molecular formula of C18H14O8. The UV spectrum showed absorption maxima at λmax 245, 255, 296, and 344 nm, which is characteristic for a flavonol structure [6]. The IR spectrum showed an absorption for hydroxyl (3423 cm−1), conjugated carbonyl (1645 cm−1), aromatic (1577 and 1481 cm−1), and ether (1132 cm−1) groups, respectively [7]. The 13C-NMR spectrum (Table 1) of 1 showed 18 carbon signals, 2 of them signals at δC 138.2 and δC 179.3, typical for a flavonol structure at C-3 and C-4 [6]. The 1H-NMR spectrum (Table 1) of 1 showed the presence of a proton signal of two aromatic units an ABX system at δH 7.68 (H-6′), 7.59 (H-2’), 6.95 (H-5′) at ring B in the aromatic region, and a singlet of an isolated aromatic proton at δH 6.54 (H-8) at ring A. The 1H-NMR spectrum of 1 also revealed the presence of two proton signals of a hydroxyl group at δH 12.88 (5-OH), δH 6.50 (7-OH); two methoxyls at δH 4.04 (6-OCH3), 3.85 (3-OCH3); and a methylenedioxy group at δH 6.08 (3′,4′-OCH2-O). The position of hydroxyl, methoxyl groups, and methylenedioxy group were confirmed based on HMQC and HMBC spectra. The long-range correlations in the HMBC spectrum of 1 showed a proton signal of a chelated hydroxyl group (δH 12.88, 5-OH) with three quaternary carbons at δC 151.8 (C-5), 130.1 (C-6), and 106.3 (C-4a). A methoxyl group at δH 4.04 was correlated with a quaternary carbon at δC 130.1 (C-6), showing that a methoxyl group was placed at C-6. The proton signal of a hydroxyl group at δH 6.50 (7-OH) correlated with a quaternary carbon at δC 155.1 (C-7), and a methine carbon at δC 93.2 (C-8), indicating that a hydroxyl group was placed at C-7 and suggesting an isolated aromatic proton at δH 6.54 at H-8. From the 1H-NMR spectrum, the presence of a proton signal of an ABX system at ring B indicated that a methylenedioxy group was placed at C-3′ and C-4′. The proton signal of a methylenedioxy group at δH 6.08 correlated with two oxyaryl carbons at δC 149.7 (C-3′) and at δC 150.0 (C-4′). Furthermore, the proton signal of a methoxyl group at δH 3.85 correlated to δC 138.2 revealed that a methoxyl group was placed at C-3. One proton signal of ABX at δH 7.59 (H-2′) showed correlations with two oxyaryl carbons—δC 155.8 (C-2), 150.0 (C-4′)—and one methine carbon at δC 123.8 (C-6′). The proton signal at δH 6.95 (H-5′) showed correlations with one quaternary carbon, δC 124.2 (C-1′), and one oxyaryl carbon at δC 149.7 (C-3′). Furthermore, the proton signal at δH 7.68 (H-6′) showed correlations with two oxyaryl carbons (δC 155.8 (C-2), 150.0 (C-4′)), and one methine carbon signal at δC 108.7 (C-2′). Based on the above spectral evidence, the structure of 1 was elucidated as 5,7-dihydroxy-3,6-dimethoxy-3′,4′-methylenedioxyflavone.

Table 1.
NMR spectroscopic data of 5,7-dihydroxy-3,6-dimethoxy-3′,4′-methylenedioxyflavone in CDCl3.
The cytotoxic activity of 1 was evaluated using cell viability in murine leukemia P-388 cells by MTT assay, exhibiting IC50 values of 48.30 μg/mL. The antioxidant activity against DPPH radical of 1 showed IC50 values of 38.68 μg/mL, which suggests that it has moderate activity.
2.1. General
Column chromatography and planar radial chromatography were carried out using silica gel 60 G 1.07734.1000 and Si gel 60 PF254 1.07749.1000 (Merck, Darmstadt, Germany). The UV spectra was measured with Shimadzu series 1800 spectrophotometer (Shimadzu, Kyoto, Japan).The IR spectra was recorded with Perkin-Elmer spectrum-100 FT-IR (Perkin-Elmer, Waltham, MA, USA). The mass spectra were recorded using a Waters LCT Premier XE (Waters, Santa Clara, CA, USA). NMR spectra were recorded on a JEOL 400 ECA spectrophotometer (JEOL, Tokyo, Japan) in CDCl3 at 400 (1H) and 100 (13C) MHz using TMS as the internal standard.
2.2. Plant Material
The leaves of M. glabra were collected in Gunung Salak, Bogor, West Java, Indonesia on March 2017. The specimen was identified at the Herbarium Bogoriense, Center of Biological Research and Development, National Institute of Science, Bogor, Indonesia.
2.3. Extraction and Isolation
The leaves of M. glabra (1.7 kg) were macerated in MeOH twice for 2 days each. After evaporating the solvent in a rotary evaporator, 210 g of pale brown semisolid was obtained. The extract was redissolved in MeOH/water (9:1) and partitioned with n-hexane (95 g) and ethyl acetate (30 g). The EtOAc extract (29 g) was subjected to vacuum liquid chromatography over silica gel and eluted with n-hexane/ethyl acetate by increasing polarity (9:1, 4:1; 7:3, 1:1, and 1:4) to give three major fractions, A–C. Fraction A (4.68 g) was separated by column chromatography eluted with n-hexane-ethyl acetate (9:1 to 7:3) to produce subfractions A1–A3. Subfraction A1 was purified by planar radial chromatography using n-hexane/CHCl3 (from 4:1 to 1:4) to yield compound 1 (20 mg).
2.4. Cytotoxic Assay
The cytotoxic activity of 1 against murine leukemia P-388 cells was evaluated according to the MTT method as previously described [8,9,10]. Artonin E was used as the positive control.
2.5. DPPH Radical Scavenging
The antioxidant activity of 1 against DPPH (2,2-diphenyl-1-picrihydrazyl) radical measured at λ 517 nm by UV spectrometer as described previously [11,12,13]. The inhibition percentage (%) of radical scavenging activity was calculated using the following equation: Inhibition (%) = (Ao − As/Ao) × 100, where Ao is the absorbance of the control reaction (containing all reagents except the active compound), and As is the absorbance of the active compound. Ascorbic acid was used as the positive control.
3. Conclusions
A new flavonol, 5,7-dihydroxy-3,6-dimethoxy-3′,4′-methylenedioxyflavone, was isolated for the first time from the leaves of M. glabra. The cytotoxic activity of 1 against murine leukemia P-388 cells showed IC50 values of 48.30 μg/mL, and the antioxidant activity against the DPPH radical showed IC50 values of 38.68 μg/mL.
Supplementary Materials
The following are available online. HRESIMS, 1H-NMR, 13C-NMR, HMQC, and HMBC spectra are reported in the Supplementary Materials as Figures S1–S5, and structure refinement parameters are in Table S1.
Author Contributions
M.T. designed the whole experiment of bioactivity and wrote the manuscript. T.S.T. researched data, analyzed the NMR and HRESIMS spectra and contributed to the manuscript, R.D.S. and U.H. designed the whole experiment. F.H., a botanist was identified of plant material. All authors read and approved the final manuscript.
Funding
This research was supported by Universitas Airlangga through Hibah Riset Mandat 2018 research.
Acknowledgments
We thanks to Ismail Rachman, a botanist was identified of plant Herbarium Bogoriense, Bogor, Indonesia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tanjung, M.; Saputri, R.D.; Wahjoedi, R.A.; Tjahjandarie, T.S. 4-Methoxy-3-(3-methylbut-2-en-1-yl)-7-((3-methylbut-2-en-1-yl)oxy) quinolin-2(1H)-one from Melicope moluccana T.G. Hartley. Molbank 2017, 2017, M939. [Google Scholar] [CrossRef]
- Nakashima, K.; Oyama, M.; Ito, T.; Akao, Y.; Witono, J.R.; Darnaedi, D.; Tanaka, T.; Murata, T.; Iinuma, M. Novel quinolinone alkaloids bearing a lignoid moiety and related constituents in the leaves of Melicope denhamii. Tetrahedron 2012, 68, 2421–2428. [Google Scholar] [CrossRef]
- Kassim, N.K.; Rahmani, M.; Ismail, A.; Sukari, M.A.; Ee, G.C.L.; Nasir, N.M.; Awang, K. Antioxidant activity-guided separation of coumarins and lignin from Melicope glabra (Rutaceae). Food Chem. 2013, 139, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, H.T. Four novel geminaly dialkylated, non-aromatic acetophenone derivatives from Melicope coodeana. Phytochem. Lett. 2012, 5, 371–375. [Google Scholar] [CrossRef]
- Sultana, N.; Hartley, T.G.; Waterman, P.G. Two novel prenylated flavanones from the aerial parts of Melicope micrococca. Phytochemistry 1999, 50, 1249–1253. [Google Scholar] [CrossRef]
- Tanjung, M.; Juliawaty, L.D.; Hakim, E.H.; Syah, Y.M. Flavonoid and stilben derivatives from Macaranga trichocarpa. Fitoterapia 2018, 126, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Marliana, E.; Astuti, W.; Kosala, K.; Hairani, R.; Tjahjandarie, T.S.; Tanjung, M. Chemical composition and anticancer activity of Macaranga hosei leaves. Asian J. Chem. 2018, 30, 795–798. [Google Scholar] [CrossRef]
- Tanjung, M.; Rachmadiarti, F.; Saputri, R.D.; Tjahjandarie, T.S. Mesuacalophylloidin, a new isoprenylated 4-phenylcoumarin from Mesua calophylloides (Ridl.) Kosterm. Nat. Prod. Res. 2018, 32, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Tanjung, M.; Hakim, E.H.; Syah, Y.M. Prenylated dihydrostilbenes from Macaranga rubiginosa. Chem. Nat. Compd. 2017, 53, 215–218. [Google Scholar] [CrossRef]
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. 5,9,11-Trihydroxy-2,2-dimethyl-10-(3′-methyl-2′-butenyl)-3-(2″-methyl-3″-butenyl)pyrano[2,3-a] xanthen-12(2H)-one from the stem bark of Calophyllum pseudomole. Molbank 2016, 2016, M906. [Google Scholar] [CrossRef]
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. Antioxidant activity of two isomeric benzoxepin derivatives from the stem bark of Bauhinia acuelata L. J. Chem. Pharm. Res. 2014, 6, 705–708. [Google Scholar]
- Tjahjandarie, T.S.; Saputri, R.D.; Tanjung, M. Methyl 2,5-Dihydroxy-4-(3′-methyl-2′-butenyl)benzoate. Molbank 2016, 2016, M892. [Google Scholar] [CrossRef]
- Tjahjandarie, T.S.; Pudjiastuti, P.; Saputri, R.D.; Tanjung, M. Antimalaria and antioxidant activity of phenolic compounds isolated from Erythrina crysta-galli L. J. Chem. Pharm. Res. 2014, 6, 786–790. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).