Abstract
The title compound (Z)-4-[2-(3,4-difluorophenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one 4 was synthesized by the reaction of ethyl 2-[2-(3,4-difluorophenyl)hydrazono]-3-oxobutanoate 3 with hydrazine hydrate. The diazotization of 3,4-difluoroaniline, followed by the treatment with ethyl acetoacetate, afforded intermediate 3. The synthesized compound 4 was characterized by FTIR, 1H-NMR, 13C-NMR and LCMS, and it showed synergistic anti-inflammatory, antiproliferative and antibacterial activities.
1. Introduction
Due to the wide spectrum of biological activities shown by pyrazolone derivatives such as analgesic, antipyretic, antirheumatic [1,2], antimicrobial [3], antiproliferative [4] and anti-inflammatory [5], these moieties are considered potent pharmacophores in medicinal chemistry. The pyrazolone molecules are well known free radical scavengers [6]. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one)—a strong free radical scavenger—is used for the treatment of patients with acute brain infarction [7]. Pyrazolones are also used as intermediates for the preparation of disazo dyes [8], analytical and polymer-bound reagents [9,10].
The anti-inflammatory activity of Nonsteroidal Anti-inflammatory Drugs (NSAIDs) is due to the inhibition of cyclooxygenase 2 (COX-2) enzyme present in the cell, which also has an isozyme form COX-1 and is involved in the housekeeping function of cytoprotection in gastric mucosa, the integrity of platelet function and the maintenance of renal perfusion [11]; whereas COX-2 causes inflammation by producing prostaglandin (PGE2) from arachidonic acid stimulation. PGE2 is one of the most widely investigated prostaglandins and its activity influences inflammation, fertility and immune modulation. The aberrant expression of COX-2 would lead to an increase in PGE2 level. It has been proven that there is a strong association between chronic inflammation and increased susceptibility to cancer [12]. The PGE2 produced by the COX-2 enzyme plays a major role in cancer by promoting cell proliferation. PGE2 signalling through both EP2 and EP4 receptors can stimulate tumour growth [13]. A molecule that inhibits the formation of PGE2 will act as a good anti-inflammatory as well as an antiproliferative agent. Hence, there is a need to develop a drug which synergistically acts as an anti-inflammatory and antitumor agent.
Certain NSAIDs like Celecoxib, which is a pyrazole derivative, selectively inhibits the COX-2 enzyme. Commonly used anti-inflammatory drugs such as phenylbutazone, propyphenazone, SC558 and so forth, possess a pyrazole moiety as a functional part of the drug. The internalized carbohydrazide bonds in the pyrazolone moiety also play a role in anti-inflammatory activity [14]. The NSAIDs with high selectivity towards COX-2 might also act as anticancer agents [15,16,17,18]. Anticancer activity of some aryl hydrazono pyrazolone derivatives against P. 388 leukaemia in mice has been reported [19].
Fluorine is the second smallest substituent and its presence often leads to increased lipid solubility enhancing the rate of absorption and transport of drugs [20]. Metabolic stability is one of the key factors in determining the bioavailability of a compound. The replacement of an oxidizable C-H group by a C-F group in a compound increases metabolic stability and binding affinity with the COX-2 enzyme. Among standard NSAIDs, Celecoxib and SC 558, which has a CF3 substituent was found to be active when compared with a CH3 substituent against the COX-2 enzyme respectively [21,22]. On the basis of the above findings, and in continuation of our interest in the synthesis, characterization and evaluation of the bioefficacy of pyrazole derivatives [6,23], the synthesis of (Z)-4-[2-(3,4-difluorophenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one (4) was contemplated, along with testing its anti-inflammatory, antiproliferative, and antimicrobial activities.
2. Results and Discussion
2.1. Chemistry
The synthesis of the new compound 4 is outlined in Scheme 1. The reaction of 3,4-difluoroanilinium chloride with ethyl acetoacetate in cold methanol afforded the intermediate ethyl 2-[(3,4-difluorophenyl)hydrazono]-3-oxobutanoate 3. Further, refluxing the solution of intermediate 3 and hydrazine hydrate in an acetic acid medium resulted in the formation of the title compound (Z)-4-[2-(3,4-difluorophenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one (4) and it was confirmed by FTIR, 1H-NMR, 13C-NMR, and LCMS analyses.
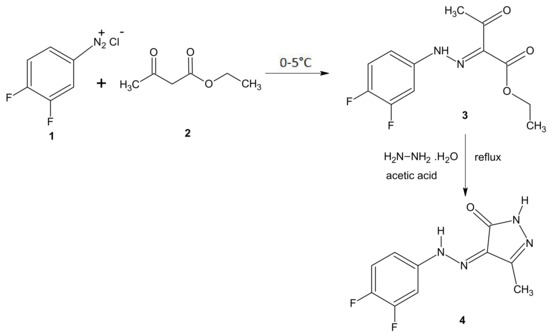
Scheme 1.
Synthesis of (Z)-4-[2-(3,4-difluorophenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one (4).
The spectral characterization of 4 is presented as follows. The IR spectrum of compound 4 showed a broad absorption band at 3250 cm−1 due to the presence of NH groups. A sharp and strong absorption band appeared at 1670 cm−1, which was assigned to the carbonyl stretching of the cyclic amide group along with a band at 1616 cm−1 (in plane) and 717 cm−1(out of plane)for NH bending. The absorption bands due to C-N and C-F stretch were seen at 1568 and 1261 cm−1, respectively. The 1H-NMR spectrum showed a singlet at δ 2.13 ppm integrating to three protons of the CH3 group attached to a pyrazolone ring. The presence of fluorine at meta and para positions led to the coupling of aromatic protons. The doublet (d) peak at δ 7.38 ppm (J = 9.2 Hz) was due to the ortho proton. Another ortho proton, adjacent to the meta fluorine, showed a doublet of doublet (dd) peak centred at δ 7.47 ppm (J = 28 Hz, H-F ortho and 8.8 Hz, H-F meta). A doublet of doublet of doublet (ddd) peak observed at δ 7.65 ppm (J = 9.6 Hz, H-F ortho, 7.2 Hz, H-H ortho and 2.4 Hz, H-F meta) was accounted for the meta proton. The singlet peak at δ 11.54 ppm integrating for a proton was due to the N-H of the pyrazolone ring. The broad peak for exocyclic NH of the hydrazono group appeared in the downfield at δ 13.03 ppm. The downfield shift could be due to the intramolecular hydrogen bond between the exocyclic N-H of the hydrazono group with the oxygen of the carbonyl group of the pyrazolone ring, which was further supported by the single crystal X-Ray diffraction structure of an analogue 5-methyl-4-(2-(2,4-dimethylphenylhydrazinylidene)-2,4-dihydro-3H-pyrazol-3-one [24]. Normally, a pyrazolone molecule exhibits tautomerism due to the presence of -NH-C=O adjacent groups in a ring, as shown in Figure 1. The formed N-H----O=C bond hinders the tautomeric phenomenon owing to the formation of a six-membered ring, which might be giving stability to the system. It is worth noting that a report has been cited for the existence of arylhydrazono tautomer, both in solid form as well as in solution for a similar analogue [25,26,27]. In the 13C-NMR spectrum, the peak at δ 12.0 ppm was assigned to methyl carbon attached to a pyrazolone ring. The chemical shifts of aromatic carbons bearing hydrogen appeared at δ 105.6 ppm (d, 2JCF = 22 Hz), δ 112.6 ppm, δ 118.7 ppm (d, 2JCF = 18 Hz). The carbon of the aromatic ring, bonded to hydrazono nitrogen, resonated at δ 139.5 ppm (d, J = 8 Hz). The meta and para fluoro substituted carbons showed doublet of doublet peaks at δ 147.0 ppm (dd, 1JCF = 241 Hz, 12 Hz), δ 150.3 ppm (dd, 1JCF = 244 Hz, 14 Hz) respectively. The pyrazolone ring carbonyl carbon resonated at δ 160.2 ppm and the other carbon atoms at δ 129.4 and δ 147.4 ppm. The downfield shift for the carbonyl group also supported the cyclic amide structure for the molecule. The mass spectrum showed a molecular ion peak at m/z 237.15 (M-1) corresponding to the molecular formula C10H8F2N4O. Elemental analysis also provided satisfactory results for the compound.
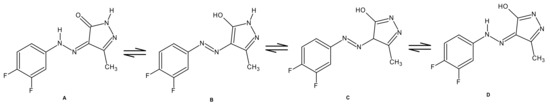
Figure 1.
Possible tautomeric structures of compound 4.
2.2. Anti-Inflammatory Studies
The synthesized compound 4 and two of the closely related compounds—namely, (Z)-4-[2-(4-fluorophenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one (PYZ-F) and (Z)-4-[2-(2,4-dimethylphenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one (PYZ-di-CH3)—were screened for in vivo anti-inflammatory activity by a carrageenan-induced paw oedema test in rats. Diclofenac sodium was administered as a standard drug for comparison. The molecule (Z)-4-[2-(3,4-difluorophenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one 4 was found to be active in inhibiting paw oedema volume by 68.29% (2 h) and 71.16% (4 h), in comparison to Diclofenac sodium, which has an inhibition of 70.12% (2 h) and 79.76% (4 h).The anti-inflammatory activity results for compounds 4, PYZ-F and PYZ-di-CH3 are summarized in Table 1.

Table 1.
Anti-inflammatory activity of compound 4 and its analogues.
2.3. Antiproliferative Studies
In vitro antiproliferative activity of the synthesized pyrazolone derivatives 4, PYZ-F and PYZ-di-CH3 was evaluated using an MTT assay against human cervix epithelial adenocarcinoma (HeLa), hepatocellular carcinoma (HepG2), and Ehrlich-Lettre ascites (EAT) cell lines. The cytotoxicity was measured at two concentrations of 10 µM and 20 µM. The compound (Z)-4-[2-(3,4-difluorophenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one 4 showed maximum antiproliferative activity with IC50 of 18 µM, 27 µM and 36 µM against HeLa, HepG2 and EAT cell lines respectively. The antiproliferative activity data of compounds 4, PYZ-F and PYZ-di-CH3 are summarized in Table 2.

Table 2.
Antiproliferative activity of compound 4 and its analogues.
2.4. Antibacterial Activity
The antibacterial activity of synthesized compounds 4, PYZ-F and PYZ-di-CH3 was assessed using the Broth micro dilution method, using four bacterial strains namely, Staphylococcus aureus, Escherichia coli (Gram positive) and Pseudomonas aeruginosa, Klebsiella pneumonia (Gram negative). Compound 4 emerged with higher efficacy, possessing a minimum inhibitory concentration (MIC) value of 26.2 µM. The MIC for nitrofurazone was less than 31.56 μM. The MIC data are given in Table 3.

Table 3.
Antibacterial activity of compound 4 and its analogues.
2.5. Molecular Docking Studies
Human glucose transporter 1 (hGLUT1) is a homologous sugar transporter that facilitates the transport of glucose across the plasma membranes. Cancerous cells grow and multiply more rapidly than normal cells, resulting in an acutely increased demand for energy. This leads to an increase in the level of hGLUT1 [28,29]. Hence hGLUT1 acts as a target for drugs in cancer therapy. The in silico molecular docking studies of compound 4 and its analogues were carried out to obtain the binding affinity with hGLUT1 (PDB: 5EQG). The docking data of compound 4 and its analogues with hGLUT1 revealed that the compounds exhibit H-bonding with one or the other amino acids in the active pockets (Figure 2).The best possible docking mode of compound 4 against the hGLUT1 active site was identified as H-bonding of the pyrazolone ring NH with Asn288 (1.92 Å), the carbonyl oxygen with Asn288 (2.06 Å) and Gln283(1.96 Å).The docking score for compound 4 was found to be −6.177 kJ·mol−1, which is comparable with the standard ligand. The docking data of analogues are summarized in Table 4. It is worth noting that compound 4 with fluorine at both para and meta positions of the phenyl ring showed an improved docking score compared with the mono fluoro derivative and unsubstituted phenyl derivative.
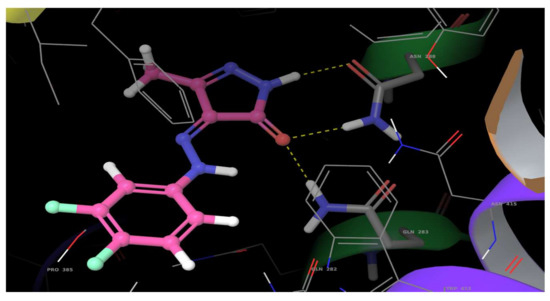
Figure 2.
Interaction of compound 4 with hGLUT1.

Table 4.
Molecular docking results compound 4 and its analogues with hGLUT1.
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
Experimental
The melting point was recorded on a melting point apparatus (Adarsh Scientific Industries, Agra, Uttar Pradesh, India) taken in an open capillary tube and was uncorrected. The purity of the compound was confirmed by thin layer chromatography using Merck silica gel 60 F254 coated aluminium plates (Merck, KGaA, Darmstadt, Germany). The IR spectrum was recorded on a Shimadzu-FTIR Infrared spectrometer in KBr (Vmax in cm−1) (Shimadzu, Kyoto, Japan). The 1H-NMR (400 MHz) spectrum was recorded on a Bruker AMX 400 spectrometer (Bruker Optik, Ettlingen, Germany), with 5 mm PABBO BB-1H TUBES at IISC, Bengaluru, Karnataka, India. The 13C-NMR (100 MHz) spectrum was recorded on a Bruker Ascend for approximately 0.03 M solutions in DMSO-d6, at the Central Instrumentation Facility, Manipal University. The mass spectrum was obtained using a Shimadzu LCMS-8030 mass spectrometer (Shimadzu Corporation, Kyoto, Japan), operating at 70 eV at Mangalore University, Mangalagangothri, Karnataka, India. The elemental analysis was carried out using a CHNSO analyser (Model: FLASH EA 1112 series, Thermo Finnigan, Thermo Electron Corporation, Milano, Italy).
3.1.2. Synthesis of (Z)-4-[2-(3,4-Difluorophenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one (4)
The intermediate ethyl 2-[2-(3,4-difluorophenyl)hydrazono]-3-oxobutanoate 3 was prepared using the reported method [30].
Hydrazine hydrate (0.75 mL, 0.015 mol) was added to the solution of ethyl 2-[2-(3,4-difluorophenyl)hydrazono]-3-oxobutanoate (2.7 g, 0.01 mol) in acetic acid and refluxed for 4–6 h. The completion of the reaction was monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature and the resulting solid product was filtered, collected, and recrystallized from methanol. Yellow solid, Yield 71%; m.p. 201–203 °C; FTIR (KBr): Vmax (cm−1), 3250 (s, NH), 1670 (C=O), 1568 (CN), 1261 (CF), 1H-NMR (400 MHz, DMSO-d6): δ ppm, 2.13 (s, 3H, pyrazolone ring CH3), 7.38 (d, 1H, J = 9.2 Hz, Ar-H), 7.47 (dd, 1H, J = 28 Hz, 8.8 Hz, Ar-H), 7.65 (ddd, 1H, J = 9.6 Hz, 7.2 Hz, 2.4 Hz, Ar-H), 11.54 (1H, s, pyrazolone NH), 13.0 (1H, bs, exocyclic NH of hydrazono group), 13C-NMR (100 MHz, DMSO-d6): δ ppm, 12.0 (CH3), 105.6 (d, 2JCF = 22 Hz), 112.6, 118.7 (d, 2JCF = 18 Hz), 129.4, 139.5 (d, J = 8 Hz), 147.4, 147.0 (dd. 1JCF = 241 Hz, 12 Hz), 150.3 (dd. 1JCF = 244 Hz, 14 Hz), 160.2 (C=O), LCMS (ESI-MS): m/z 237.15 (M-1). C H N analysis; calculated for C10H8F2N4O: C, 50.42; H, 3.39; N, 23.52. Found: C, 50.35; H, 3.30; N, 23.59.
The analogues of compound 4—(Z)-4-[2-(2,4-dimethylphenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)–one (PYZ-di-CH3) and (Z)-4-[2-(4-fluorophenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one (PYZ-F)—were prepared by the reported procedure [24,27].
3.2. Anti-Inflammatory Activity
In vivo anti-inflammatory activity of compound 4 and its analogues was carried out by carrageenan induced paw oedema test in rats. Diclofenac sodium (20 mg/kg) was administered as a standard drug for comparison. Rats were divided into 12 groups of 6 each. Group I was treated with tween-80 (1%) suspension which served as the vehicle control. Group II to VI were treated with the suspension of the test compounds at a dose of 50 mg/kg. Group VII was administered with the standard drug Diclofenac sodium. After 30 min, the sub planter region of the left hind paw of rats was injected with 0.1 mL of carrageenan (1% w/v). The paw volume was measured after 2 and 4 h using the mercury displacement technique with the help of a plethysmometer [31].
3.3. Antiproliferative Studies
HeLa, HepG2, and EAT cell lines were cultured and an MTT assay was used for cytotoxicity. Value of IC50 for the compound 4 and its analogues was determined by plotting the percent cytotoxicity index [32]. Assessment of cell mortality was done by trypan blue exclusion method. Cells (5 × 104 cells/well) were seeded in six well plates prior to the addition of pyrazolones. The cells were incubated with different doses of compounds along with 1% DMSO as a solvent control. Cultures were harvested and monitored for cell number by counting cell suspensions using a hemocytometer. Cell mortality was checked before and after treatment with compounds using the trypan blue exclusion method [33].
3.4. Antibacterial Activity
The in vitro antibacterial activity of compound 4 and its analogues was carried out using the Broth micro dilution method described by NCCLS in a 96-well microtitre plate to get the minimum inhibitory concentration of the test compounds (MIC) [34]. The in vitro antibacterial activity was carried out against a 24 h culture of four bacterial strains—namely Staphylococcus aureus, Escherichia coli, (Gram positive) and Pseudomonas aeruginosa, Klebsiella pneumoniae (Gram negative).
The synthesized compounds at different concentrations were prepared by dissolving in DMSO. Overnight broth cultures of standard microorganisms were prepared in MHB and the final bacterial load was adjusted to the 5.0 × 105 CFU/mL photometrically. The test compounds at different concentrations were added to the corresponding wells containing MHB with prepared standard microbial strains and the multiwell plates were incubated aerobically at 37 °C for 24 h. Positive and negative controls were included in the study. Nitrofurazone was used as a standard drug for comparison of antibacterial activity. The MIC was the lowest concentration of the test compound which completely inhibits the growth of the microorganism.
3.5. Molecular Docking Studies
The molecular docking studies were conducted at Mangalore University on Intel i5 powered Acer Veriton Workstations using the Small-Molecule Drug Discovery Suite 2017-1, Schrödinger, LLC, New York, NY, 2017. The respective proteins were imported into the Maestro interface directly from the Protein Data Bank (PDB). The proteins were minimized using the Protein Preparation Wizard. The missing loops and missing side chains were filled using Prime. Epik was used to generate het states at pH 7.0 ± 2.0. The compounds, along with the inhibitor, were prepared using LigPrep. The inhibitor molecule was selected in order to generate a grid around it using Receptor Grid Generation. The prepared ligands were docked into the protein using Glide. Extra Precision (XP) mode was used during the docking studies. The computational pharmacokinetics studies (Ligand based ADME/Tox Prediction) were performed using QikProp (Schrödinger).
4. Conclusions
A compound (Z)-4-[2-(3,4-difluorophenyl)hydrazono]-3-methyl-1H-pyrazol-5(4H)-one (4) was synthesized, characterized and screened for in vivo anti-inflammatory, in vitro antiproliferative and antibacterial activities. The biological activity of compound 4 was compared with its analogues PYZ-F and PYZ-di-CH3. The molecular docking studies of 4 with hGLUT1 revealed that the presence of fluorine at both the para and meta positions increased binding affinity. These findings suggested that compound 4 is a promising molecule with synergistic anti-inflammatory, antiproliferative and antibacterial properties.
Supplementary Materials
Copies of FTIR, 1H-NMR, 13C-NMR and LCMS analyses are enclosed. Figure S1. IR spectrum of compound 4. Figure S2. 1H-NMR spectrum of compound 4. Figure S3. 13C-NMR spectrum of compound 4. Figure S4. LC and Mass spectrum of compound 4.
Acknowledgements
The authors are thankful to IISc, Bangalore and Central Instrumentation Facility, Manipal University for NMR, USIC, Mangalore University for providing the facility for spectral analysis. The authors Billava Jayappa Mohan and Balladka Kunhanna Sarojini thank the Department of Atomic Energy (DAE)/Board of Research and Nuclear Sciences (BRNS), Government of India, for providing financial assistance under the BRNS Project (Grant No. 2011/34/20-BRNS/0846).
Author Contributions
All authors contributed equally to all activities: design, synthesis, biological study, results interpretation and manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ei Ashry, E.S.H.; Awad, L.F.; Ibrahim, E.I.; Bdeewy, O.K. Synthesis of antipyrine derivatives derived from dimedone. Chin. J. Chem. 2007, 25, 570. [Google Scholar] [CrossRef]
- Uramaru, N.; Shigematsu, H.; Toda, A.; Eyanagi, R.; Kitamura, S.; Ohta, S. Design, synthesis, and pharmacological activity of nonallergenic pyrazolone-type antipyretic analgesics. J. Med. Chem. 2010, 53, 8727–8733. [Google Scholar] [CrossRef] [PubMed]
- Samshuddin, S.; Narayana, B.; Sarojini, B.K.; Khan, M.T.H.; Yathirajan, H.S.; Darshan Raj, C.G.; Raghavendra, R. Antimicrobial, analgesic, DPPH scavenging activities and molecular docking study of some 1,3,5-triaryl-2-pyrazolines. Med. Chem. Res. 2012, 21, 2012–2022. [Google Scholar] [CrossRef]
- Rao, B.S.; Akberali, P.M.; Holla, B.S.; Sarojini, B.K. Synthesis and studies on some new fluorine containing hydroxypyrazolines and 1H pyrazoles-as possible antiproliferative agents. J. Pharmacol. Toxicol. 2008, 3, 102–110. [Google Scholar]
- Bansal, E.; Srivastava, V.K.; Kumar, A. Synthesis and anti-inflammatory activity of 1-acetyl-5-substituted aryl-3-(β-aminonaphthyl)-2-pyrazolines and β-(substituted aminoethyl) amidonaphthalenes. Eur. J. Med. Chem. 2001, 36, 81–92. [Google Scholar] [CrossRef]
- Sarojini, B.K.; Vidyagayatri, M.; Darshanraj, C.G.; Bharath, B.R.; Manjunatha, H. DPPH Scavenging Assay of Novel 1, 3-disubstituted-1H-pyrazol-5-ols and their in silico Studies on Some Proteins Involved in Alzheimers Disease Signalling Cascade. Lett. Drug Des. Discov. 2010, 7, 214–224. [Google Scholar] [CrossRef]
- Higashi, Y.; Jitsuikia, D.; Chayama, K.; Yoshizumia, M. Edaravone (3-methyl-1-Phenyl-2-pyrazolin-5-one), anovel free radical scavenger, for treatment of cardiovascular diseases. Recent Patents Cardiovasc. Drug Discov. 2006, 1, 85–93. [Google Scholar] [CrossRef]
- Karcı, F.; Karcı, F. The synthesis and solvatochromic properties of some novel heterocyclic disazo dyes derived from barbituric acid. Dyes Pigment. 2008, 77, 451–456. [Google Scholar] [CrossRef]
- Desai, K.C.; Indorwala, N.S. Environmentally sustainable analytical reagent like 1-(2’-chloro-5’-sulphophenyl)-3-methyl-4-azo-(2′′-carboxy-5′′-sulphonic acid)-5-pyrazolone as a spectrophotometric reagent for Mn (II). J. Environ. Res. Dev. 2012, 6, 1024–1028. [Google Scholar]
- Byun, J.W.; Lee, D.H.; Lee, Y.S. Preparation of polymer-bound pyrazolone active esters for combinatorial chemistry. Tetrahed. Lett. 2003, 44, 8063–8067. [Google Scholar] [CrossRef]
- Clària, J. Cyclooxygenase-2 biology. Curr. Pharma. Design 2003, 9, 2177–2190. [Google Scholar] [CrossRef]
- Allavena, P.; Signorelli, M.; Chieppa, M.; Erba, E.; Bianchi, G.; Marchesi, F.; Olimpio, C.O.; Bonardi, C.; Garbi, A.; Lissoni, A.; et al. Anti-inflammatory Properties of the Novel Antitumour Agent Yondelis (Trabectedin): Inhibition of Macrophage Differentiation and Cytokine Production. Cancer Res. 2005, 65, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Smartt, H.J.M.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.R.S.; Salvador, R.R.S. Pyrazole Carbohydrazide Derivatives of Pharmaceutical Interest. Pharmaceuticals 2012, 5, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Azam, F.; Rghigh, A.M.; Gbaj, A.; Zetrini, A.E. Structure-based design, synthesis, molecular docking, and biological activities of 2-(3-benzoyl phenyl) propanoic acid derivatives as dual mechanism drugs. J. Pharm. Bioallied Sci. 2012, 4, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.I.; DuBois, R.N. NSAIDs and cancer prevention: Targets downstream of COX-2. Annul. Rev. Med. 2007, 58, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; Henley, S.J.; Patrono, C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J. Natl. Cancer. Inst. 2002, 94, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A. Nonsteroidal anti-inflammatory drugs and cyclooxygenase inhibition in the gastrointestinal tract: a trip from peptic ulcer to colon cancer. Am. J. Med. Sci. 2009, 338, 96–106. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.F.; Conalty, M.L. Proceedings of the Royal Irish Academy Section B. Biol. Geol. Chem. Sci. 1980, 80B, 385–394. [Google Scholar]
- Filler, R.; Saha, R. Fluorine in medicinal chemistry: a century of progress and a 60-year retrospective of selected highlights. Future Med. Chem. 2009, 1, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Bohm, H.J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Muller, K.; Sander, U.O.; Stahl, M. Fluorine in Medicinal Chemistry. Chem. Biol. Chem. 2004, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.D.; Talley, J.J.; Bertenshaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Malecha, J.W.; Miyashiro, J.M.; et al. Synthesis and Biological Evaluation of the 1.5 Diarylpyrazole Class of Cyclooxygenase-2 Inhibitors: Identification of 4-[5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazole-1-yl] benzenesulfonamide (SC-58634, Celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Vidyagayatri, M.; Darshanraj, C.G.; Sarojini, B.K.; Sreenivasa, S.; Jayaramu, M. Synthesis, characterization, evaluation of antiproliferative and antimicrobial properties of new pyrazole derivatives. J. Pharm. Res. 2011, 4, 2787–2790. [Google Scholar]
- Sarojini, B.K.; Mohan, B.J.; Narayana, B.; Yathirajan, H.S.; Jasinski, J.P.; Butcher, R.J. (Z)-4-[2-(2,4-Dimethylphenyl)hydrazinylidene]-3-methylpyrazol-5(1H)-one. Acta Cryst. E 2013, E69, o532. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Quah, C.K.; Kalluraya, B. 4-[2-(4-Chlorophenyl)hydrazinylidene]-3-methyl-1H-pyrazol-5(4H)-one. Acta Cryst. E 2011, E67, o2670. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.K.; Hassan, M.A.; Mohamed, M.M.; El-Sayed, A.M. Metal salt-catalyzed diazocoupling of 3-substituted-1H-pyrazol-2-in-5-ones in aqueous medium. Dyes Pigment. 2005, 66, 241–245. [Google Scholar] [CrossRef]
- Hassan, A.E.A.; Moustafa, A.H.; Tolbah, M.M.; Zohdy, H.F.; Haikal, A.Z. Synthesis and Antimicrobial Evaluationof Novel Pyrazolones and Pyrazolone Nucleosides. Nucleos. Nucleot. Nucl. 2012, 31, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science. 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, K.; Finer-Moore, J.S.; Pedersen, B.P.; Caboni, L.; Waight, A.; Hillig, R.C.; Bringmann, P.; Heisler, I.; Muller, T.; Siebeneicher, H.; et al. Mechanism of inhibition of human glucose transporter GLUT1 is conserved between cytochalasin B and phenylalanine amides. Proc. Natl. Acad. Sci. USA 2016, 113, 4711–4716. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.P.; Rajput, S.S. Synthesis and Microbial Screening of Seven Membered Heterocyclic ring Compounds from 1,2-diaminobenzene. Int. J. Pharm. Tech. Res. 2009, 1, 900–904. [Google Scholar]
- Arunkumar, S.; Ilango, K.; Manikandan, R.S.; Ramalakshmi, N. Synthesis and anti-inflammatory activity of some novel pyrazole derivatives of gallic acid. Euro. J. Chem. 2009, 6, S123–S128. [Google Scholar] [CrossRef][Green Version]
- Cetin, Y.; Bullerman, L.B. Cytotoxicity of Fusarium mycotoxins to mammalian cell cultures as determined by the MTT bioassay. Food. Chem. Toxicol. 2005, 43, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.; Bertoglio, J.; Breard, J. Lysosomal and mitochondrial pathways in miltefosine-induced apoptosis in U937 cells. Apoptosis 2007, 12, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).