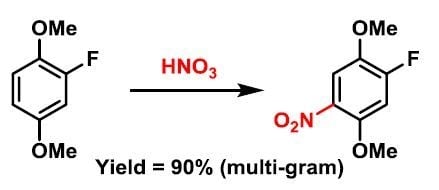
1-Fluoro-2,5-dimethoxy-4-nitrobenzene
Abstract
:1. Introduction
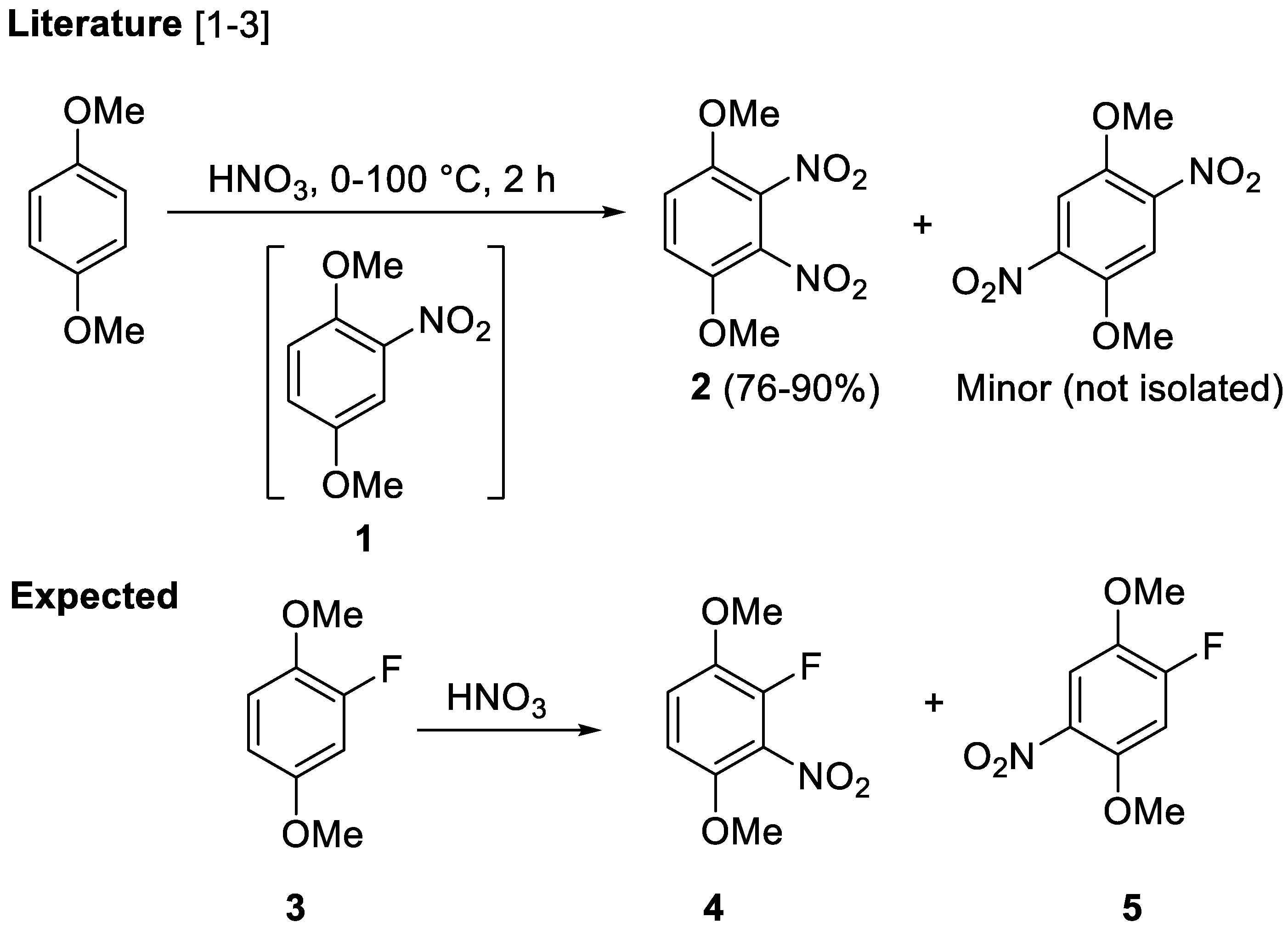
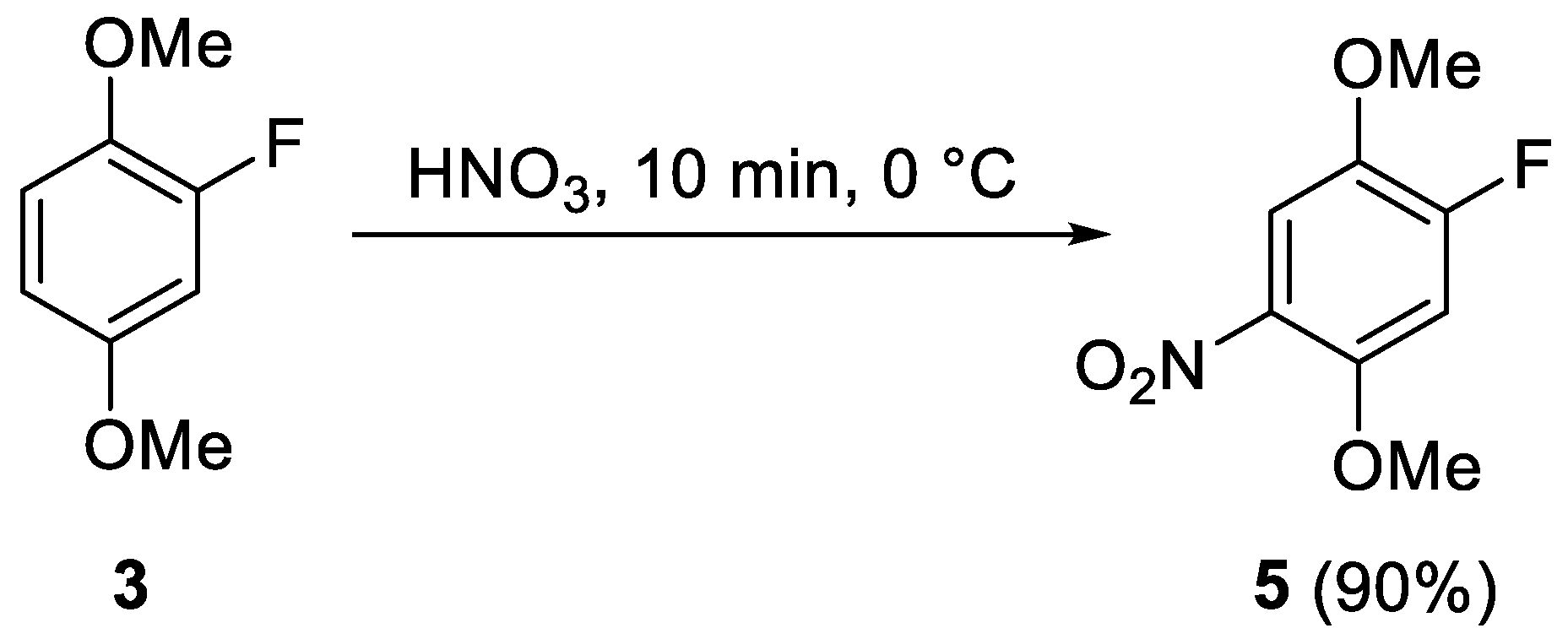
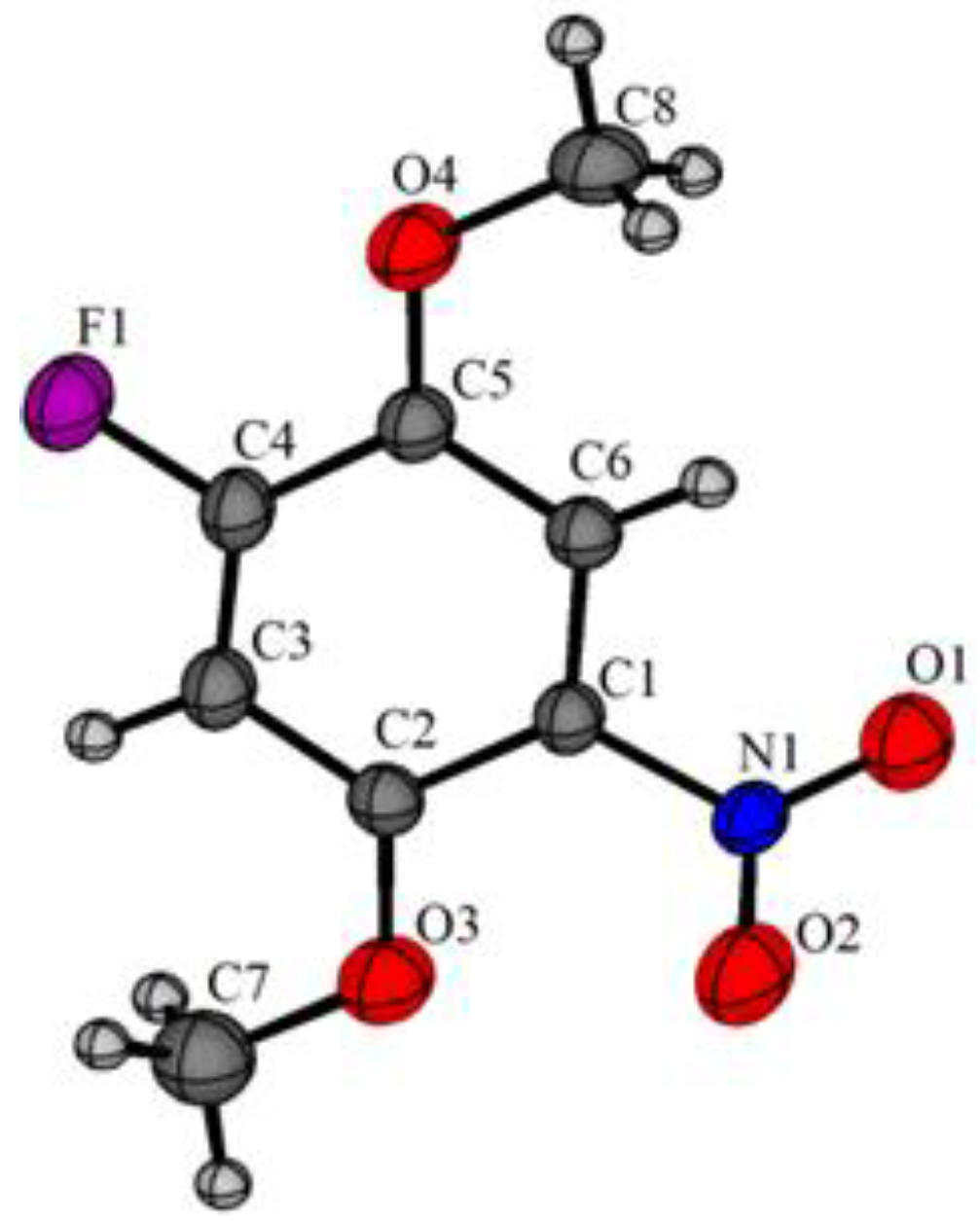
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 1-Fluoro-2,5-dimethoxy-4-nitrobenzene (5)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taleb, A.; Alvarez, F.; Nebois, P.; Walchshofer, N. An improved methodology for the preparation of 4,7-dimethoxy-1H-benzimidazole, a key intermediate in the synthesis of 1-alkyl-1H-benzimiadzole-4,7-diones. Heterocycl. Commun. 2006, 12, 111–114. [Google Scholar] [CrossRef]
- Hammershøj, P.; Reenberg, T.K.; Pittelkow, M.; Nielsen, C.B.; Hammerich, O.; Christensen, J.B. Synthesis and properties of 2,3-Dialkynyl-1,4-benzoquinones. Eur. J. Org. Chem. 2006, 12, 2786–2794. [Google Scholar] [CrossRef]
- Gurry, M. The benign synthesis of bioactive heterocycles using photochemistry and hydrogen peroxide with hydrohalic acids. Ph.D. Thesis, NUI Galway, Galway, Ireland, 2016. [Google Scholar]
- Lynch, M.; Hehir, S.; Kavanagh, P.; Leech, D.; O’Shaughnessy, J.; Carty, M.P.; Aldabbagh, F. Synthesis by radical cyclization and cytotoxicity of highly potent bioreductive alicyclic ring fused [1,2-a]benzimidazolequinones. Chem. Eur. J. 2007, 13, 3218–3226. [Google Scholar] [CrossRef] [PubMed]
- Fahey, K.; O’Donovan, L.; Carr, M.; Carty, M.P.; Aldabbagh, F. The influence of the aziridinyl substituent of benzimidazoles and benzimidazolequinones on toxicity towards normal and Fanconi anaemia cells. Eur. J. Med. Chem. 2010, 45, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.; Gurry, M.; Keane, L.-A.J.; Aldabbagh, F. Greener synthesis using hydrogen peroxide in ethyl acetate of alicyclic ring-fused benzimidazoles and anti-tumour benzimidazolequinones. Tetrahedron Lett. 2017, 58, 3565–3567. [Google Scholar] [CrossRef]
- Chemspace. Available online: https://chem-space.com (accessed on 24 January 2018).
- Laali, K.K. Nitro and nitroso transformations in superacids. Coord. Chem. Rev. 2000, 210, 47–71. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- McArdle, P. Oscail, a program package for small-molecule single-crystal crystallography with crystal morphology prediction and molecular modelling. J. Appl. Crystallogr. 2017, 50, 320–326. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sweeney, M.; McArdle, P.; Aldabbagh, F. 1-Fluoro-2,5-dimethoxy-4-nitrobenzene. Molbank 2018, 2018, M984. https://doi.org/10.3390/M984
Sweeney M, McArdle P, Aldabbagh F. 1-Fluoro-2,5-dimethoxy-4-nitrobenzene. Molbank. 2018; 2018(1):M984. https://doi.org/10.3390/M984
Chicago/Turabian StyleSweeney, Martin, Patrick McArdle, and Fawaz Aldabbagh. 2018. "1-Fluoro-2,5-dimethoxy-4-nitrobenzene" Molbank 2018, no. 1: M984. https://doi.org/10.3390/M984
APA StyleSweeney, M., McArdle, P., & Aldabbagh, F. (2018). 1-Fluoro-2,5-dimethoxy-4-nitrobenzene. Molbank, 2018(1), M984. https://doi.org/10.3390/M984