A Novel Disulfide-Containing Polycationic Amphiphile: 1,28-Di[(cholest-5-en-3β-yl)disulfanyl]-4,25-dioxo-3,8,12,17,21,26-hexaazaoctacosane Tetrahydrochloride
Abstract
:1. Introduction
2. Results and Discussion
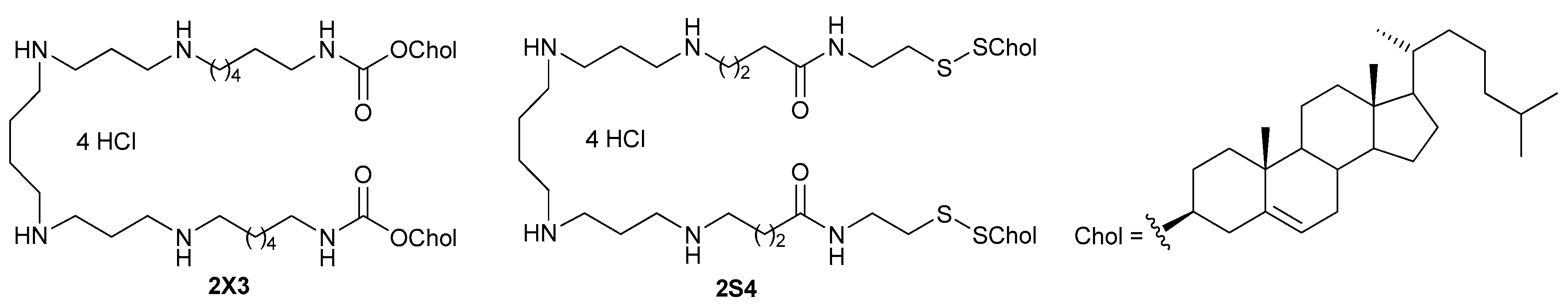
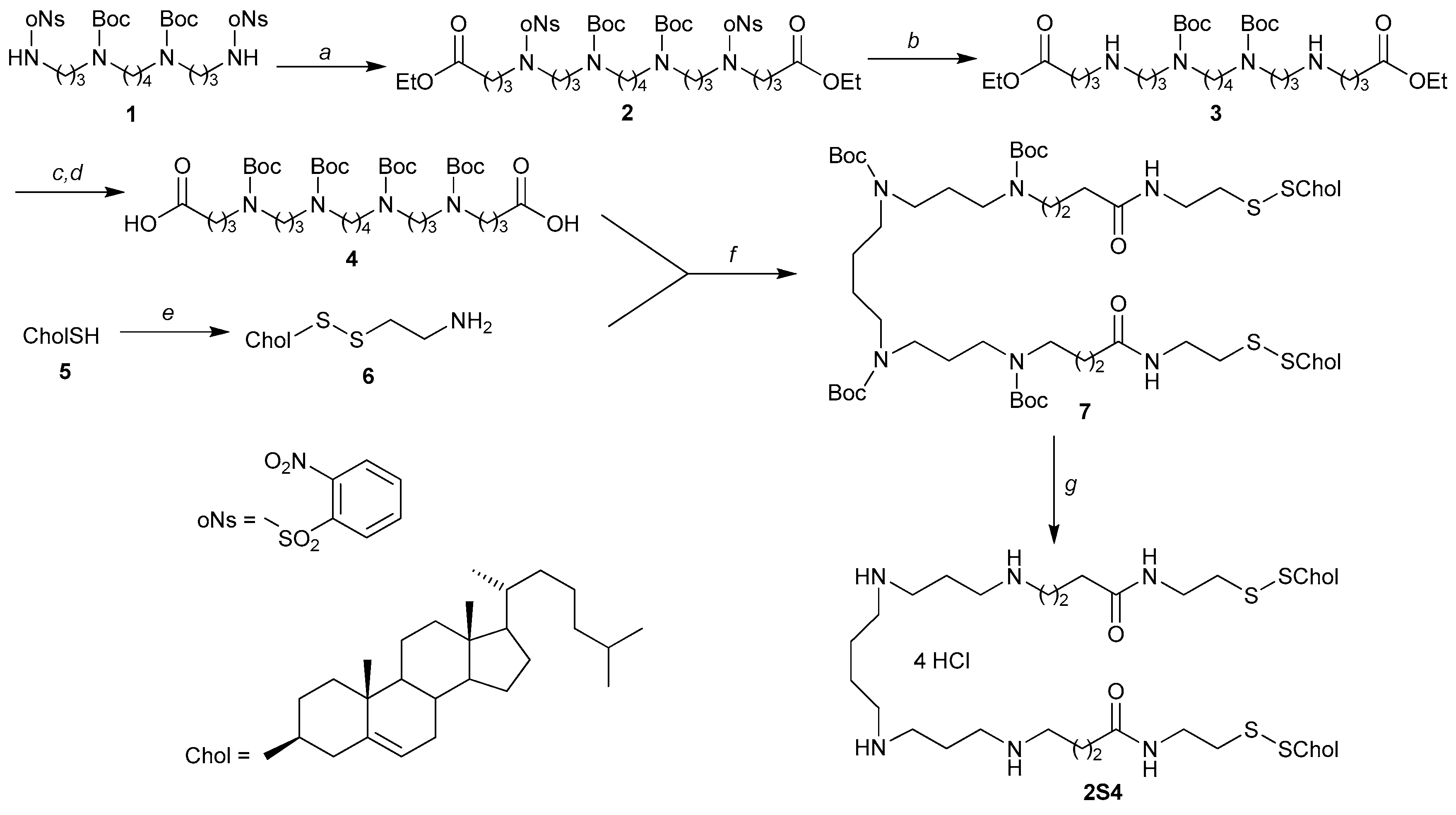
2.1. Synthesis of 2S4
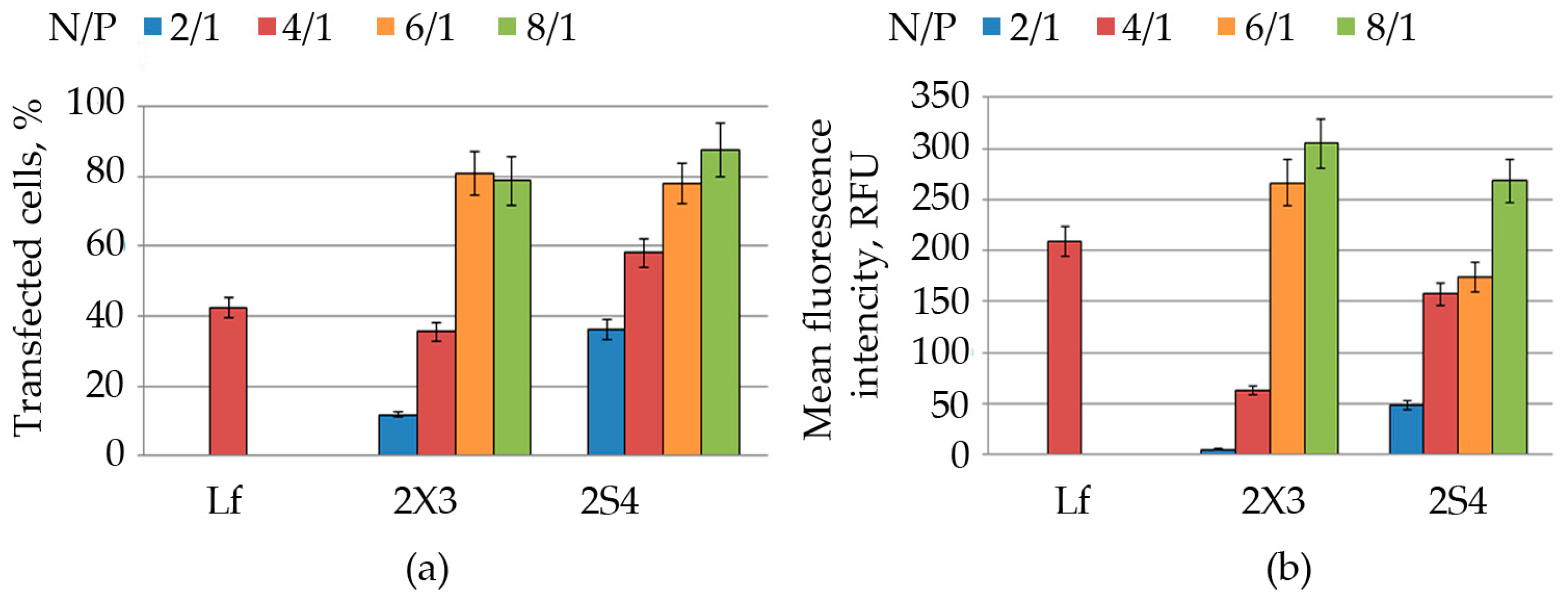
2.2. Cationic Liposomes and Their Transfection Efficiency
3. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alton, E.W.; Armstrong, D.K.; Ashby, D.; Bayfield, K.J.; Bilton, D.; Bloomfield, E.V.; Boyd, A.C.; Brand, J.; Buchan, R.; Calcedo, R.; et al. A randomised, double-blind, placebo-controlled trial of repeated nebulisation of non-viral cystic fibrosis transmembrane conductance regulator (CFTR) gene therapy in patients with cystic fibrosis. Effic. Mech. Eval. 2016, 3, 1–210. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S. Endgame: Glybera Finally Recommended for Approval as the First Gene Therapy Drug in the European Union. Mol. Ther. 2012, 20, 1831–1832. [Google Scholar] [CrossRef] [PubMed]
- Delalande, A.; Gosselin, M.-P.; Suwalski, A.; Guilmain, W.; Leduc, C.; Berchel, M.; Jaffrès, P.-A.; Baril, P.; Midoux, P.; Pichon, C. Enhanced Achilles tendon healing by fibromodulin gene transfer. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Markov, O.O.; Mironova, N.L.; Maslov, M.A.; Petukhov, I.A.; Morozova, N.G.; Vlassov, V.V.; Zenkova, M.A. Novel cationic liposomes provide highly efficient delivery of DNA and RNA into dendritic cell progenitors and their immature offsets. J. Control. Release 2012, 160, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Bodles-Brakhop, A.M.; Draghia-Akli, R. DNA vaccination and gene therapy: Optimization and delivery for cancer therapy. Expert Rev. Vaccines 2008, 7, 1085–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, L. Lipid nanoparticles for gene delivery. In Advances in Genetics; Huang, L., Liu, D., Wagner, E., Eds.; Academic Press Inc.: San Diego, CA, USA, 2014; Volume 88, pp. 13–36. ISBN 9780128001486. [Google Scholar]
- Sanchez, A.; Pensado, A. Current strategies for DNA therapy based on lipid nanocarriers. Expert Opin. Drug Deliv. 2014, 11, 1721–1731. [Google Scholar] [CrossRef]
- Guo, X.; Szoka, F.C. Chemical approaches to triggerable lipid vesicles for drug and gene delivery. Acc. Chem. Res. 2003, 36, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, H.; Tachibana, C.; Winther, J.R. Monitoring disulfide bond formation in the eukaryotic cytosol. J. Cell Biol. 2004, 166, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-T.; Yi, W.-J.; Su, R.-C.; Liu, Q.; Zhao, Z.-G. Reducible amino acid based cationic lipids as highly efficient and serum-tolerant gene vectors. Chempluschem 2016, 81, 125–134. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Liang, H.; Jiang, Q.; Ke, B.; Nie, Y.; Peehl, D.; Knox, S.; Zhang, Q. Disulfide modified self-assembly of lipopeptides with arginine-rich periphery achieve excellent gene transfection efficiency at relatively low nitrogen to phosphorus ratios. J. Mater. Chem. B 2017, 5, 1482–1497. [Google Scholar] [CrossRef]
- Sheng, R.; Luo, T.; Zhu, Y.; Li, H.; Sun, J.; Chen, S.; Sun, W.; Cao, A. The intracellular plasmid DNA localization of cationic reducible cholesterol-disulfide lipids. Biomaterials 2011, 32, 3507–3519. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Kondaiah, P.; Bhattacharya, S. Effect of the nature of the spacer on gene transfer efficacies of novel thiocholesterol derived gemini lipids in different cell lines: A structure-activity investigation. J. Med. Chem. 2008, 51, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Fraix, A.; Le Gall, T.; Berchel, M.; Denis, C.; Lehn, P.; Montier, T.; Jaffrès, P.-A. Cationic lipophosphoramidates with two disulfide motifs: Synthesis, behaviour in reductive media and gene transfection activity. Org. Biomol. Chem. 2013, 11, 1650. [Google Scholar] [CrossRef] [PubMed]
- Byk, G.; Wetzer, B.; Frederic, M.; Dubertret, C.; Pitard, B.; Jaslin, G.; Scherman, D. Reduction-sensitive lipopolyamines as a novel nonviral gene delivery system for modulated release of DNA with improved transgene expression. J. Med. Chem. 2000, 43, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Maslov, M.A.; Kabilova, T.O.; Petukhov, I.A.; Morozova, N.G.; Serebrennikova, G.A.; Vlassov, V.V.; Zenkova, M.A. Novel cholesterol spermine conjugates provide efficient cellular delivery of plasmid DNA and small interfering RNA. J. Control. Release 2012, 160, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Petukhov, I.A.; Maslov, M.A. Synthesis of polycationic lipids based on cholesterol and spermine. Rus. Chem. Bull. Int. Ed. 2010, 59, 260–268. [Google Scholar] [CrossRef]
- Fukuyama, T.; Jow, C.K.; Cheung, M. 2- and 4-Nitrobenzenesulfonamides: Exceptionally versatile means for preparation of secondary amines and protection of amines. Tetrahedron Lett. 1995, 36, 6373–6374. [Google Scholar] [CrossRef]
- Nagy, P. Kinetics and Mechanisms of Thiol–Disulfide Exchange Covering Direct Substitution and Thiol Oxidation-Mediated Pathways. Antioxid. Redox. Signal. 2013, 18, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, W.; Mackay, J.; Szoka, F.C. Thiocholesterol-based lipids for ordered assembly of bioresponsive gene carriers. Mol. Ther. 2005, 11, 409–417. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchkov, P.A.; Shmendel, E.V.; Andreeva, V.D.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. A Novel Disulfide-Containing Polycationic Amphiphile: 1,28-Di[(cholest-5-en-3β-yl)disulfanyl]-4,25-dioxo-3,8,12,17,21,26-hexaazaoctacosane Tetrahydrochloride. Molbank 2018, 2018, M981. https://doi.org/10.3390/M981
Puchkov PA, Shmendel EV, Andreeva VD, Morozova NG, Zenkova MA, Maslov MA. A Novel Disulfide-Containing Polycationic Amphiphile: 1,28-Di[(cholest-5-en-3β-yl)disulfanyl]-4,25-dioxo-3,8,12,17,21,26-hexaazaoctacosane Tetrahydrochloride. Molbank. 2018; 2018(1):M981. https://doi.org/10.3390/M981
Chicago/Turabian StylePuchkov, Pavel A., Elena V. Shmendel, Valeria D. Andreeva, Nina G. Morozova, Marina A. Zenkova, and Mikhail A. Maslov. 2018. "A Novel Disulfide-Containing Polycationic Amphiphile: 1,28-Di[(cholest-5-en-3β-yl)disulfanyl]-4,25-dioxo-3,8,12,17,21,26-hexaazaoctacosane Tetrahydrochloride" Molbank 2018, no. 1: M981. https://doi.org/10.3390/M981
APA StylePuchkov, P. A., Shmendel, E. V., Andreeva, V. D., Morozova, N. G., Zenkova, M. A., & Maslov, M. A. (2018). A Novel Disulfide-Containing Polycationic Amphiphile: 1,28-Di[(cholest-5-en-3β-yl)disulfanyl]-4,25-dioxo-3,8,12,17,21,26-hexaazaoctacosane Tetrahydrochloride. Molbank, 2018(1), M981. https://doi.org/10.3390/M981




