Abstract
The title ester (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 4-aminobutyrate hydrochloride was obtained in 96% yield via Steglich esterification. The structure of the target compound was established by FTIR, HR-MS, 1H-NMR, 13C-NMR spectral analysis, and single crystal X-ray diffraction study. Single crystals of the title ester suitable for X-ray investigation were obtained by slow evaporation of the methanolic solution at room temperature. The purity of compound was assessed using HPLC coupled to mass spectrometry.
1. Introduction
Since the identification, cloning, and characterization of the transient receptor potential (TRP) ion channels, special attention has been focused on terpenes and their derivatives as against/antagonist of aforementioned pharmacological targets. Cyclic terpene alcohol menthol is one of TRP modulators possessing antinociceptive and local anesthetic effects [1,2]. Among four pairs of optical isomers (−)-menthol—also known as l-menthol with (1R,2S,5R) configuration—occurs most widely in the nature and has the greatest cooling activity [3,4]. Besides binding to TRP channels, l-menthol was found to act as positive allosteric modulators of γ-aminobutyric acid (GABA)A receptors [5].
Based on the foregoing, the combination of l-menthol residues with GABA into one molecule is reasonable to enhance the effect of each component. Thus, the current note is devoted to the detailed description and determination of ester structure based on menthol and GABA—(1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 4-aminobutyrate hydrochloride.
2. Results and Discussion
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 4-aminobutyrate hydrochloride was synthesized via Steglich esterification with N,N′-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) as a catalyst in dichloromethane, as shown in Scheme 1. Synthesized ester was isolated in 96% yield as white solid soluble in methanol and dimethyl sulfoxide and fully characterized by 1H-NMR, FTIR-spectroscopy and FAB-, ESI-mass spectrometry. Additionally, the HPLC analysis was carried out to determine the purity of the title compound. For this purpose, reversed-phase HPLC method with isocratic elution of methanol:ammonium formate buffer was applied. The observed retention time for the ester was 2.676 min with 98% of purity.
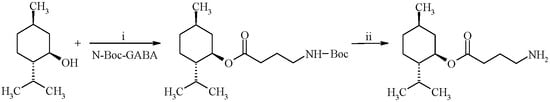
Scheme 1.
Synthesis of (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 4-aminobutyrate. Reagents and conditions: (i) DMAP, CH2Cl2, 20 °C, 10 min; DCC, 0 °C, 30 min; rt, 10 h; (ii) HCl, CH3COOH. The ester was prepared as hydrochloride.
The FAB-MS spectra of the title ester displays the protonated molecular ion peak [M + H]+ at m/z 242. The HRMS (ESI-TOF) revealed an ion peak of the compound at m/z 242.2134 [M + H]+, thus suggesting a molecular formula C14H28NO2 (calc. 242.2120). The FTIR spectra of ester exhibits absorption bands of N–H bonds (3021 cm−1), C=O ester groups (1721 cm−1), C–O at 1151–1201 cm−1, and alkyl C–H. The 1H-NMR spectral data contain resonance signals described by their chemical shift, integration, and multiplicity that are in full agreement with the presented molecular formula. The 1H-NMR spectrum of synthesized compound contains the proton H-1 of cyclohexane ring resonated at δ 4.53–4.59 ppm as a triplet of doublets. The methyl group at C-5 is observed as a doublet at δ 0.67 ppm with SSCC J = 6.53 Hz. Signals of axial and equatorial ring protons are also presented in the 1H-NMR spectrum, their position and multiplicity correspond to similar signals in the spectrum of l-menthol. Thus, according to 1H-NMR analysis, the initial configuration of l-menthol is preserved.
Additionally l-configuration of menthol was confirmed by X-ray diffraction analysis (see Figure 1). Single crystals of the title compound suitable for X-ray investigation were obtained by slow evaporation of the methanolic solution at room temperature.
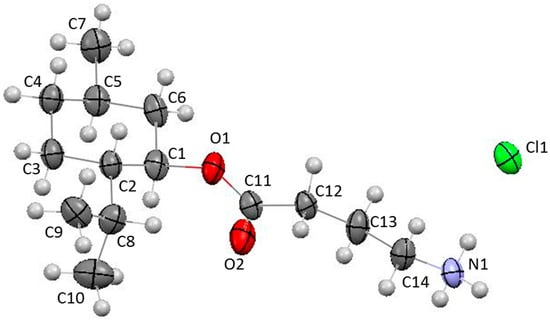
Figure 1.
The molecular structure of (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 4-aminobutyrate hydrochloride according to X-ray diffraction data. All atoms are represented by 50% probability ellipsoids.
According to the X-ray diffraction data, the synthesized compound is a chloride of organic cation. The positive charge of the cation is localized on the amino group as indicated by three hydrogen atoms that were found from the electron density difference map; in addition, this fact is proved by elongation of N1-C14 bond to 1.486(4) Å compared with the average value of 1.469 Å [6]. The title compound crystallizes in non-centrosymmetric space group indicating the presence of only one enantiomer in the crystal. Configuration of chiral centers at С1, С2, and С5 atoms (R, S, and R, respectively) was simultaneously determined by calculating the Flack parameter (−0.04(10)). The saturated cycle adopts the chair conformation (puckering parameters are [7]: S = 1.16, θ = 0.52°, ψ = 49.2°). Under these conditions, atoms C2 and С5 deviate to different sides from mean-square plane of the remaining atoms of this cycle (deviation amounts to 0.67 Å and −0.69 Å, respectively). Methyl and isopropyl substituents are located in the equatorial positions (the C1-C6-C5-C7 and С6-С1-С2-С8 torsion angles are −180.0(3)° and −178.5(3)°); the isopropyl group is rotated in such a way that the С1-С2-С8-H8 torsion angle is 49.7(4)°. Ester substituent is also equatorially oriented and its carboxyl moiety is almost orthogonally rotated to the cycle (the С5-С6-С1-О1 and С6-С1-О1-С11 torsion angles are −175.8(3)° and −104.8(4)°). Aminoalkyl fragment has a transoid conformation (the torsion angles are: С1-О1-С11-С12 −177.5(3)°, О1-С11-С12-С13 −156.6(3)°, С11-С12-С13-С14 −151.8(3)°, С12-С13-С14-N1 −178.8(3)°).
In the crystal, each cation is bound to three chloride ions by intermolecular hydrogen bonds N1-H1a…Cl1′ (−x, y − 0.5, 1 − z) H…Cl 2.49 Å N-H…Cl 145°; N1-H1b…Cl1’ (−x, 0.5 + y, 1 − z) H…Cl 2.24 Å N-H…Cl 162°; N1-H1c…Cl1′ H…Cl 2.24 Å N-H…Cl 168° (see Figure 2). As a result, endless hydrogen-bonded chains along the [010] crystallographic direction are formed. Neighboring chains are bound by weaker С-Н…Cl intermolecular hydrogen bonds: C9-H9a…Cl1′ (1 + x, y − 1, z) H…Cl 2.89 Å C-H…Cl 145°; C12-H12a…Cl1′ (1 + x, y, z) H…Cl 2.68 Å C-H…Cl 150°.
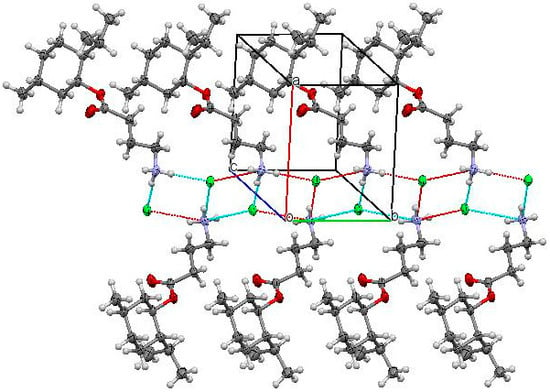
Figure 2.
Infinite chain formed by (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 4-aminobutyrate hydrochloride along the [010] crystallographic direction in the crystal phase.
3. Materials and Methods
3.1. General Information
The following chemicals were used as obtained from their commercial suppliers: l-menthol, DMAP, GABA (Acros Organics, Geel, Belgium; Darmstadt, Germany), DCC, di-tert-butyl dicarbonate (TCI, Philadelphia, PA, USA). Boc-protected GABA was not obtained commercially and has been synthesized according to the literature procedure [8]. Structure of the obtained compound was established by 1H-NMR spectroscopy on a AVANCE DRX 500 (500 MHz) instrument (Bruker, Davis, CA, USA) and by 13C-NMR spectroscopy on Varian-Mercury 400 spectrometer (Varian Inc., Palo Alto, CA, USA) using DMSO-d6 as a solvent and TMS as an internal standard. FAB mass spectrum was obtained on a VG 70-70EQ mass spectrometer (VG Analytical Ltd., Manchester, UK) equipped with Xe ion gun (8 kV); the sample was mixed with m-nitrobenzyl-alcohol matrix. High-resolution mass spectrometry (HRMS) was performed on a 6530 Accurate Mass quadrupole time of flight (Q-TOF) spectrometer (Agilent, Santa Clara, CA, USA) using ESI (electrospray ionization) coupled to an Agilent 1260 Infinity HPLC system. IR spectrum was measured with a Frontier FT-IR spectrometer (Perkin-Elmer, Hopkinton, MA, USA) using KBr pellets. The purity and identity of the compound were monitored by TLC on Merck-made (TLC Silica gel 60 F254) plates (Darmstadt, Germany).
3.2. Synthesis of (1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 4-Aminobutyrate Hydrochloride
To a stirred solution of l-menthol (0.5 g, 3.2 mmol) in CH2Cl2 (20 mL) at room temperature Boc-protected GABA (0.662 g, 3.26 mmol) and 4-dimethylaminopyridine (DMAP) (0.097 g, 0.794 mmol) were added. The reaction mixture was cooled to 0 °C, stirred for 10 min, and N,N′-dicyclohexylcarbodiimide (DCC) was added dropwise (0.727 g, 3.53 mmol). Stirring was continued for 30 min, then the flask was gradually warmed to room temperature and the stirring continued for additional 10 h. Reaction completion was monitored by TLC. Reaction mixture was filtered, the filtrate was diluted to 100 mL and washed with 1 M aqueous HCl, 10% aqueous NaHCO3, and water. Deprotection of the N-Boc group was carried out using HCl/CH3COOH according to the literature procedure [9]. Recrystallization from methanol afforded the title compound (96% yield, white solid). M.p. 222–224 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 0.67 (d, J = 6.53 Hz, 3Н, СН3-7), 0.81–0.84 (m, 7Н, СН3-9,10 + Н-4а), 0.87–0.93 (m, 1Н, Н-6а), 0.96–1.01 (m, 1Н, Н-2), 1.27–1.32 (m, 1Н, Н-5), 1.40 (m, 1Н, Н-3а), 1.59 (m, 2Н, Н-3е + Н-4е), 1.76 (t, 2Н, γ-СН2), 1.82 (d, J = 14.55 Hz, 1Н, Н-6е), 2.37 (m, 2Н, β-СН2), 2.74 (t, 2Н, α-СН2), 4.53–4.59 (td, 1Н, Н-1). FTIR (KBr, νmax, cm−1): 3021 (NH3+); 2957–2868 (C–H); 1721 (C=O); 1573, 1604 (NH3+). 13C-NMR (100 MHz, DMSO-d6) δ: 172.4 (C), 74.1 (CH), 47.1 (CH), 41.2 (CH2), 38.7 (CH2), 34.4 (CH2), 31.4 (CH), 31.1 (CH2), 26.4 (CH2), 23.6 (CH2), 23.0 (CH3), 21.0 (CH3), 16.9 (CH3). MS (FAB) m/z: 242 [M + H]+. HRMS (ESI-TOF) calculated for C14H28NO2 [M + H]+ 242.2120, found 242.2134.
3.3. X-ray Structural Analysis
The crystals of the title compound (C14H28NO2Cl) are monoclinic, colorless. At −173 °C: a = 7.8922(6), b = 5.9765(4), c = 17.047(2) Å, β = 97.382(9)°, V = 797.4(1) Å3, Mr = 277.82, Z = 2, space group Р21, Dcalc = 1.157 g/cm3, μ(MoKα) = 0.236 mm−1, F(000) = 304. The unit cell parameters and intensities of 7,671 reflections were measured on an Xcalibur diffractometer using MoKα radiation, a Charge Coupled Device (CCD) detector, graphite monochromator, and ω-scanning to 2θmax = 60°. The structure was solved by the direct method using the SHELXTL program package [10]. The positions of the hydrogen atoms were found from the electron density difference map and refined using the “rider” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl and protonated amino groups, and n = 1.2 for the other hydrogen atoms). The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 = 0.130 using 3591 reflections (R1 = 0.065 using 2318 reflections with F > 4σ (F), Sc0.947). The final atomic coordinates and crystallographic data for molecules have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition numbers CCDC 1565559.
4. Conclusions
Steglich esterification was successfully applied to synthesize the title compound, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 4-aminobutyrate hydrochloride, followed by structure confirmation via 1H-NMR, 13C-NMR, FTIR, MS, and X-ray diffraction analysis.
Supplementary Materials
Copies of the 1H-NMR, 13C-NMR, FTIR, ESI mass spectra and HPLC chromatogram are available online http://www.mdpi.com/1422-8599/2017/3/M956.
Supplementary File 1Author Contributions
I.K. conceived and designed the experiments; M.N. performed the synthesis and analyzed NMR and FTIR spectral data; S.S. performed the measurement and analysis of the X-ray experiments; G.M. performed HRMS and HPLC analysis; I.R. carried out FAB characterization of the compound; all authors contributed to writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A.; Ghelardini, C. Menthol: A natural analgesic compound. Neurosci. Lett. 2002, 322, 145–148. [Google Scholar] [CrossRef]
- Galeotti, N.; Ghelardini, C.; Mannelli, L.; Mazzanti, G.; Baghiroli, L.; Bartolini, A. Local anaesthetic activity of (+)- and (−)-menthol. Planta Med. 2001, 67, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Heidelberg, Germany, 2007; pp. 1–648. [Google Scholar]
- Eccles, R.; Weber, O. Common Cold; Birkhäuser: Basel, Switzerland, 2009; pp. 1–354. [Google Scholar]
- Lau, B.K.; Karim, S.; Goodchild, A.K.; Vaughan, C.W.; Drew, G.M. Menthol enhances phasic and tonic GABAA receptor-mediated currents in midbrain periaqueductal grey neurons. Br. J. Pharmacol. 2014, 171, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Burgi, H.-B.; Dunitz, J.D. Structure Correlation; Wiley-VCH: Weinheim, Germany, 1994; pp. 1–936. [Google Scholar]
- Zefirov, N.S.; Palyulin, V.A.; Dashevskaya, E.E. Stereochemical studies. XXXIV. Quantitative description of ring puckering via torsional angles—The case of 6-membered rings. J. Phys. Org. Chem. 1990, 3, 147–158. [Google Scholar] [CrossRef]
- Cheedarala, R.K.; Sunkara, V.; Park, J.W. Facile synthesis of second-generation dendrons with an orthogonal functional group at the focal point. Synth. Commun. 2009, 39, 1966–1980. [Google Scholar] [CrossRef]
- Pozdnev, V.F. Activation of carboxylic acids with pyrocarbonates. Esterification of N-acylamino acids with secondary alcohols using di-tret-butylpyrocarbonate—Pyridine as the condensing reagents. Russ. J. Bioorg. Chem. 1985, 11, 725–732. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).