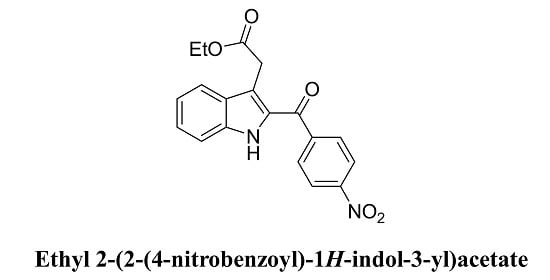
Ethyl 2-[2-(4-Nitrobenzoyl)-1H-indol-3-yl]acetate
Abstract
:1. Introduction
2. Experimental Section
2.1. General Information
2.2. Syntheis of ethyl 2-[2-(4-nitrobenzoyl)-1H-indol-3-yl]acetate(4)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine Indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Gul, W.; Hamann, M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–543. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, K.C.; Chattopadhyay, S.K. Heterocycles in Natural Product Synthesis; Wiley-VCH: Weinheim, Germany, 2011; pp. 221–265. [Google Scholar]
- Brancale, A.; Silvestri, R. Indole, a core nucleus for potent inhibitors of tubulin polymerization. Med. Res. Rev. 2007, 27, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Folkes, L.K.; Wardman, P. Oxidative activation of indole-3-acetic acids to cytotoxic species—A potential new role for plant auxins in cancer therapy. Biochem. Pharm. 2001, 61, 129–136. [Google Scholar] [CrossRef]
- Wardman, P. Indole-3-acetic acids and horseradish peroxidase: A new prodrug/enzyme combination for targeted cancer therapy. Curr. Pharm. Des. 2002, 8, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Kim, C.; Kim, S.-G. One-pot synthesis of functionalized 2,3-disubstituted indolines via K2CO3-promoted cascade Michael/aza-cyclization reactions. Bull. Korean Chem. Soc. 2015, 36, 417–420. [Google Scholar] [CrossRef]
- Yu, M.; Kim, S.-G. Stereoselective synthesis of cis-2,3-disubstituted indolines via an aza-alkylation/Michael cascade reaction. Tetrahedron Lett. 2015, 56, 7034–7037. [Google Scholar] [CrossRef]
- Kim, A.; Yu, M.; Sim, J.-T.; Kim, S.-G. One-pot synthesis of 2-acylindole-3-acetylketones via domino aza-alkylation/Michael reaction using o-aminophenyl α,β-unsaturated ketones followed by desulfonative dehydrogenation. Bull. Korean Chem. Soc. 2016, 37, 1529–1532. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Cruz-Lopez, O.; Carrion, M.D.; Cara, C.L.; Balzarini, J.; Fabbri, E.; Gambari, R. Synthesis and biological evaluation of a series of 2-(3,4,5-trimethoxybenzoyl)-indol-3-yl acetic acid derivatives as potential agents against human leukemia K562 cells. Lett. Drug Des. Descov. 2008, 5, 214–220. [Google Scholar] [CrossRef]
- Nakao, K.; Murata, Y.; Koike, H.; Uchida, C.; Kawamura, K.; Mihara, S.; Hayashi, S.; Stevens, R.W. Synthesis of 2-acylindole-3-acetic acids: A novel base-mediated indole synthesis. Tetrahedron Lett. 2003, 44, 7269–7271. [Google Scholar] [CrossRef]
- Caron, S.; Vazquez, E. Efficient synthesis of [6-chloro-2-(4-chlorobenzoyl)-1H-indol-3-yl]acetic acid, a novel COX-2 inhibitor. J. Org. Chem. 2003, 68, 4104–4107. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Kim, S.-G. Ethyl 2-[2-(4-Nitrobenzoyl)-1H-indol-3-yl]acetate. Molbank 2017, 2017, M945. https://doi.org/10.3390/M945
Choi S, Kim S-G. Ethyl 2-[2-(4-Nitrobenzoyl)-1H-indol-3-yl]acetate. Molbank. 2017; 2017(3):M945. https://doi.org/10.3390/M945
Chicago/Turabian StyleChoi, Sunyoung, and Sung-Gon Kim. 2017. "Ethyl 2-[2-(4-Nitrobenzoyl)-1H-indol-3-yl]acetate" Molbank 2017, no. 3: M945. https://doi.org/10.3390/M945
APA StyleChoi, S., & Kim, S.-G. (2017). Ethyl 2-[2-(4-Nitrobenzoyl)-1H-indol-3-yl]acetate. Molbank, 2017(3), M945. https://doi.org/10.3390/M945