Abstract
The title compound was prepared by electrophilic aromatic substitution of 7-bromo-1-methyl-2-phenyl-1H-indole with NCTS (N-cyano-N-phenyl-p-toluenesulfonamide). The structural identity of the title compound was proven by elemental analysis and spectroscopic methods (IR, NMR, APCI-MS). Purity was assessed by two independent HPLC methods.
1. Introduction
In recent years, inhibitors of tumor-related protein kinases were established as major therapeutic options for the treatment of various human cancers [1,2]. In the context of our studies directed to identify new protein kinase inhibitors based on the indole structure [3,4,5,6,7], we were interested in the synthesis of the title compound 7-bromo-1-methyl-2-phenyl-1H-indole 3-carbonitrile (3).
2. Results and Discussion
The preparation of the starting material 1 was accomplished employing methods published in the literature [8,9]. The cyanation of indoles in the 3 position has been described earlier by the use of chlorosulfonyl isocyanate [10]. However, our attempt to apply this reaction to the starting compound 1 failed, leading only to traces of the desired product 3. We therefore used NCTS (N-cyano-N-phenyl-p-toluenesulfonamide) (2) [11] as an alternative reagent for the introduction of the nitrile group by electrophilic aromatic substitution [12] (Scheme 1). This reaction is carried out by heating the reagents in 1,2-dichloroethane in a closed vessel. Boron trifluoride diethyl etherate as a Lewis acid catalyzes the reaction by activating the cyanating reagent NCTS. The nitrile group acts as an electrophile and is transferred to position 3 of the indole. This method furnished the title compound with high regioselectivity in moderate yield. The crude product which was obtained after work-up was purified by crystallization from methanol.
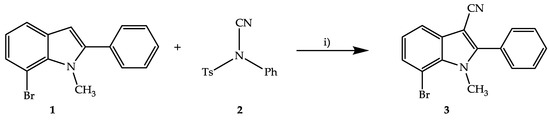
Scheme 1.
Synthesis of 7-bromo-1-methyl-2-phenyl-1H-indole-3-carbonitrile (3) with N-cyano-N-phenyl-p-toluenesulfonamide (NCTS) (2). Reagents and conditions: i) BF3·OEt2, 1,2-dichloroethane, closed vessel, bath temperature: 100 °C, 24 h.
Purity was assessed by two independent HPLC methods (isocratic and gradient) and proved to be sufficient for biological studies (>95%). The IR spectrum displayed the characteristic absorption maximum for the C≡N stretching vibration (2212 cm−1).
3. Materials and Methods
3.1. Materials
The reagents and solvents were purchased from Acros Organics, Geel, Belgium. The reagents were used without further purification. 1,2-Dichloroethane was purchased in extra dry quality. Purified water was used for sodium hydroxide solution and dilute hydrochloric acid as well as for washing. For HPLC analysis reagents of proper quality were used.
3.2. Instrumentation
The melting points were detected in open-glass capillaries on an electric variable heater (Electrothermal IA 9100, Bibby Scientific, Stone, UK). The infrared spectra were recorded on a Thermo Nicolet FT-IR 200 spectrometer (Thermo Nicolet, Madison, WI, USA) using KBr pellets. The 13C-NMR and the 1H-NMR spectra were recorded on a Bruker Avance AV II-600 spectrometer (Bruker Corporation, Billerica, MA, USA) (at the NMR Laboratories of the Chemical Institutes of the Technische Universität Braunschweig) in DMSO-d6. Chemical shifts are presented in relation to TMS (δ = 0 ppm). C nuclei were assigned based on results of 13C-DEPT135 experiments. HPLC was performed on a VWR Hitachi Chromaster system (Hitachi High Technologies Corporation, Tokyo, Japan) (DAD detector: 5430; column oven: 5310; pump: 5110; autosampler: 5260; column: Merck LiChroCART 125-4, LiChrospher 100 RP-18 (5 μm) (Merck, Darmstadt, Germany); isocratic eluent: acetonitrile/water mixture 70:30; gradient elution: concentration acetonitrile 0–2 min: 10%; 2–12 min: 10% → 90% (linear) 12–20 min: 90%; elution rate: 1.000 mL/min; detection wavelength: 254 nm and 280 nm (isocratic), 254 nm (gradient); overall run time: 15 min (isocratic), 20 min (gradient); ts = dead time; tms = total retention time). For mass spectrometry an expressionL CMS spectrometer was used with APCI source coupled with ASAP (atmospheric solids analysis probe) (Advion, Ltd., Harlow, UK). The elemental analysis was performed on a CE Instruments Flash EA® 1112 Elemental Analyzer (Thermo Quest, San Jose, CA, USA). TLC: Polygram SIL G/UV254, 0.2 mm thickness (Macherey-Nagel, Düren, Germany).
3.3. Synthesis
7-Bromo-1-methyl-2-phenyl-1H-indole (121 mg, 0.423 mmol) (2) and NCTS (115 mg, 0.423 mmol) (3) were dissolved in 1,2-dichloroethane (1 mL) in an argon flushed reaction vessel and boron trifluoride diethyl etherate (200 µL, 1.58 mmol) was added. The solution was stirred in a closed vessel for 24 h in a bath heated to 100 °C. After dilution with 1,2-dichloroethane (10 mL), the solution was washed with aqueous sodium hydroxide (85 g/L), hydrochloric acid (73 g/L) and water (5 mL each) successively. Evaporation of the organic phase gave a greenish solid substance. The crude product was purified by dissolving in boiling methanol (12 mL) and crystallized by cooling the mixture to 2 °C and filtering the solids, yielding greenish needles (54 mg, 41%).
M.p.: 176–178 °C; TLC (petrol ether/ethyl acetate 4:1): Rf = 0.41; 1H-NMR (600 MHz, DMSO-d6): δ (ppm) = 4.02 (s, 3H, CH3), 7.23 (t, 1H, J = 7.9 Hz, C(5)H), 7.61 (dd, 1H, J = 7.7/1.0 Hz, ArH), 7.62–7.70 (m, 6H, ArH); 13C-NMR (151 MHz, DMSO-d6): δ (ppm) = 35.3 (CH3), 118.4, 123.8, 129.0 (2C), 129.1, 130.1 (2C), 130.4 (CH), 85.0, 104.6, 115.5, 128.0, 129.7, 132.9, 150.2 (C); IR (KBr) (cm−1): 2212 (C≡N), 1556 (ar C=C), 1479, 1463, 1440, 1413, 1393, 1353, 1296, 1201, 1130, 1064, 812, 784, 700; MS (APCI. rel. intensity) m/z (%): 311 ([M + H]+, 100%); HPLC (AUC%): 99.34% at 254 nm, 99.65% at 280 nm, tms = 4.66 min, ts (DMSO) = 1.27 min (isocratic); 98.70% at 254 nm, tms = 13.27 min, ts (DMSO) = 1.22 min (gradient); Anal. calculated for C16H11BrN2 (311.18): C, 61.76; H, 3.56; N, 9.00. Found: C, 61.79; H, 3.47; N, 8.87. 1H- and 13C-NMR spectra are reported in the supplementary materials as Figures S1 and S2.
Supplementary Materials
The following are available, Figure S1: 1H-NMR spectrum of 3, Figure S2: 13C-NMR spectrum of 3.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
This work was supported by the state of Lower Saxony, Germany, by a Georg-Christoph-Lichtenberg-Stipend in the doctorate program “Processing of Poorly Soluble Drugs at Small Scale” (to R.M.).
Author Contributions
R.M.: Experimental synthetic work, synthesis planning, literature research, HPLC, IR, MS and NMR interpretation; M.B., J.L.: Experimental synthetic work, HPLC, IR, MS and NMR interpretation, writing of manuscript; C.K.: Synthesis planning, literature research, writing of manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Anal. | elemental analysis |
| APCI-MS | atmospheric pressure chemical ionisation mass spectrometry |
| HPLC | high performance liquid chromatography |
| IR | infrared spectrometry |
| NMR | nuclear magnetic resonance |
| TLC | thin layer chromatography |
| TMS | tetramethylsilane |
References
- Roskoski, R., Jr. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol. Res. 2015, 100, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kunick, C.; Egert-Schmidt, A.-M. Young, competitive, successful: A short history of protein kinase inhibitors. Pharm. Unserer Zeit 2008, 37, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Tolle, N.; Kunick, C. Paullones as inhibitors of protein kinases. Curr. Top. Med. Chem. 2011, 11, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Falke, H.; Bumiller, K.; Harbig, S.; Masch, A.; Wobbe, J.; Kunick, C. 2-tert-Butyl-5,6,7,8,9,10-hexahydrocyclohepta[b]indole. Molbank 2011, 2011, M737. [Google Scholar] [CrossRef]
- Schmidt, S.; Preu, L.; Lemcke, T.; Totzke, F.; Schächtele, C.; Kubbutat, M.H.G.; Kunick, C. Dual IGF-1R/SRC inhibitors based on a N′-aroyl-2-(1H-indol-3-yl)-2-oxoacetohydrazide structure. Eur. J. Med. Chem. 2011, 46, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- Meine, R.; Falke, H.; Kötz, J.; Schweda, S.I.; Kunick, C. 7-Iodo-1H-indole-3-carbonitrile. Molbank 2015, 2015, M869. [Google Scholar] [CrossRef]
- McGrath, C.F.; Pattabiraman, N.; Kellogg, G.E.; Lemcke, T.; Kunick, C.; Sausville, E.A.; Zaharevitz, D.W.; Gussio, R. Homology model of the CDK1/cyclin B complex. J. Biomol. Struct. Dyn. 2005, 22, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Tellers, D.M.; Streckfuss, E.C.; Chen, C.-Y.; Davies, I.W. [1,2]-Aryl migration in the synthesis of substituted indoles. Scope, mechanism, and high throughput experimentation. Tetrahedron 2009, 65, 3285–3291. [Google Scholar] [CrossRef]
- Islam, S.; Larrosa, I. “On water”, phosphine-free palladium-catalyzed room temperature C-H arylation of indoles. Chem. Eur. J. 2013, 19, 15093–15096. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Dhar, D.N.; Suri, S.C. Reaction of indoles with chlorosulphonyl isocyanate; a versatile route to 3-substituted indoles. Synthesis 1978, 1978, 374–376. [Google Scholar] [CrossRef]
- Kurzer, F. Cyanamides. Part I. The synthesis of substituted arylsulphonylcyanamides. J. Chem. Soc. 1949, 1034–1038. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Wang, J. Lewis acid catalyzed direct cyanation of indoles and pyrroles with N-cyano-N-phenyl-p-toluenesulfonamide (NCTS). Org. Lett. 2011, 13, 5608–5611. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).