4-Acetyl-2-hydroxy-2,5-dimethylfuran-3(2H)-one
Abstract
:1. Introduction
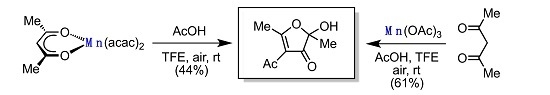
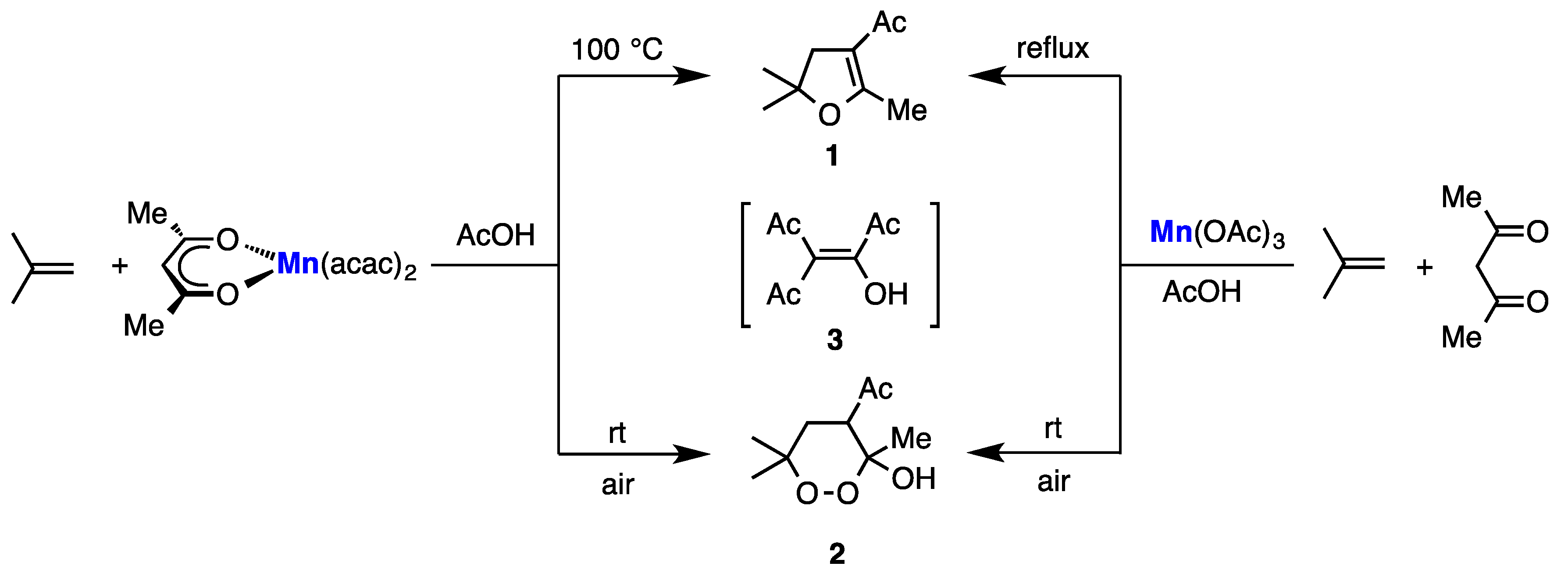
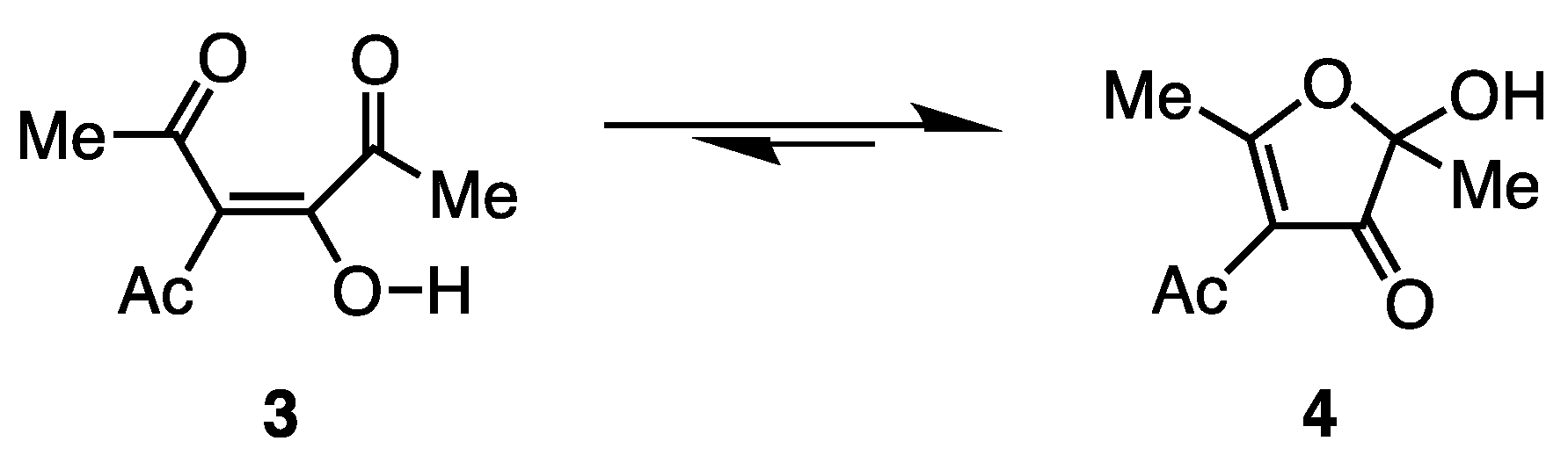
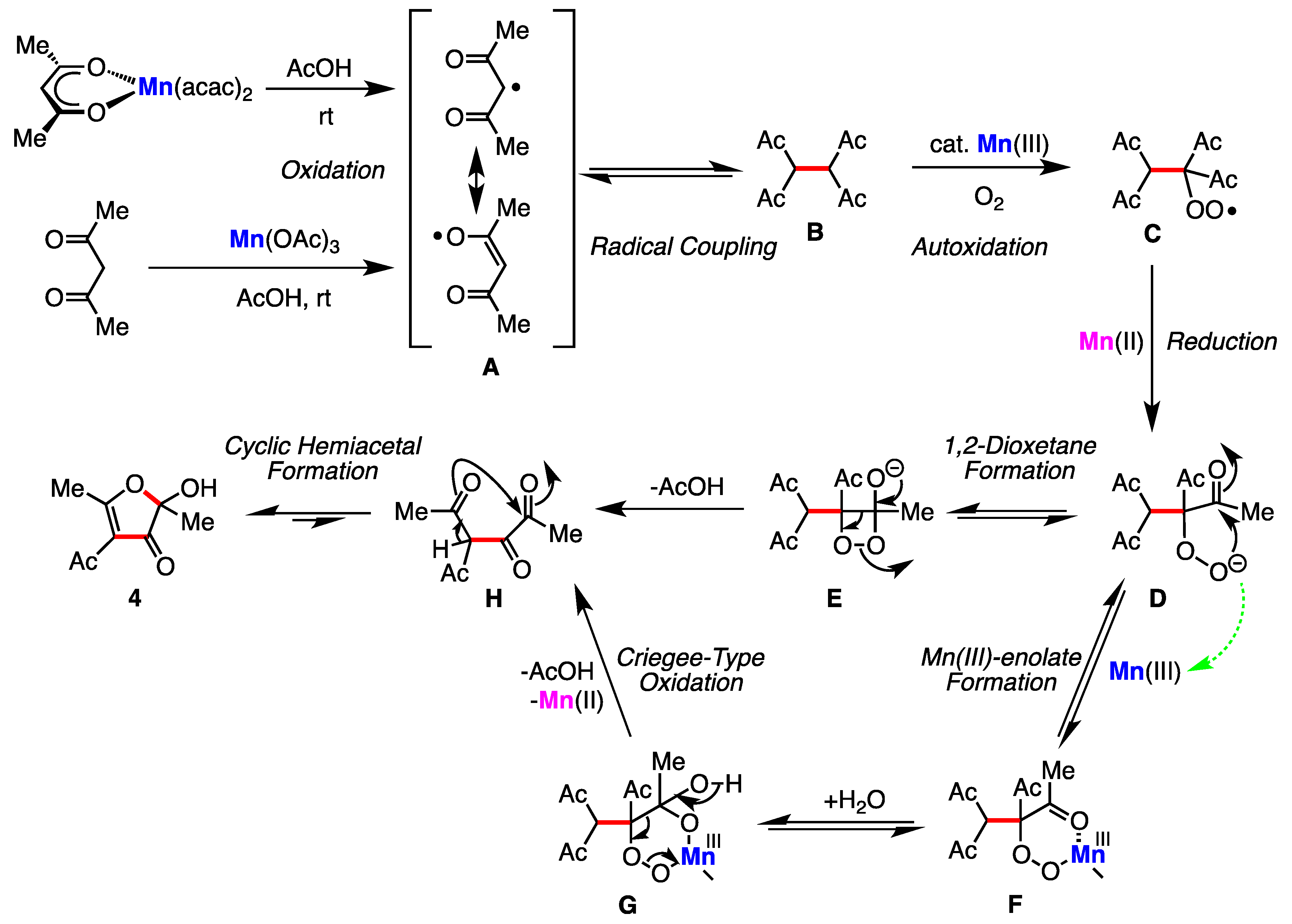
2. Results
3. Discussion
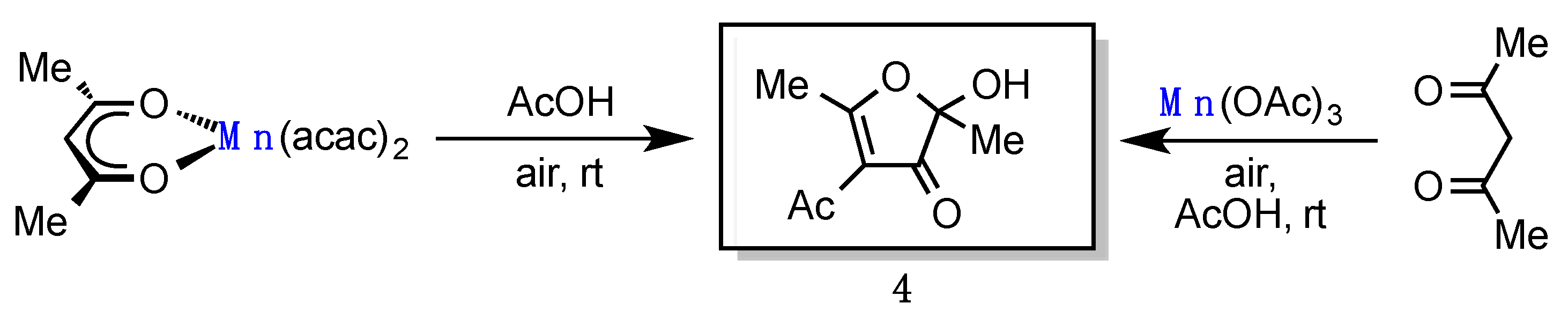
4. Experimental
4-Acetyl-2-hydroxy-2,5-dimethylfuran-3(2H)-one (4) [2,3]
4-Acetyl-2,5-dimethyl-3-oxo-2,3-dihydrofuran-2-yl Acetate (5) [2]
4-Acetyl-2-methoxy-2,5-dimethylfuran-3(2H)-one (6)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nishino, H. The Facile Synthesis of Dihydrofurans by the Oxidation of Olefins with Tris(2,4-pentanedionato)manganese(III). Bull. Chem. Soc. Jpn. 1985, 58, 1922–1927. [Google Scholar] [CrossRef]
- Nishino, H.; Tategami, S.; Yamada, T.; Korp, J.D.; Kurosawa, K. Formation of 1,2-Dioxanes by the Use of Tris(2,4-pentanedionato)manganese(III) or Manganese(III) Acetate. Bull. Chem. Soc. Jpn. 1991, 64, 1800–1809. [Google Scholar] [CrossRef]
- Nishino, H. Direct Diacetylmethylation of Aromatic Compounds with Tris(2,4-pentanedionato)manganese(III). Bull. Chem. Soc. Jpn. 1986, 59, 1733–1739. [Google Scholar] [CrossRef]
- Chechina, N.V.; Zubar, V.V.; Omelchenko, I.V.; Kolos, N.N. One-pot synthesis of new derivatives of 3,4-dihydropyrimidinone, and substituted imidazolin-2-ones. ARKIVOC 2015, 293–304. [Google Scholar]
- Ni, F.; Yang, Y.; Shu, W.-M.; Ma, J.-R.; Wu, A.-X. Brønsted acid promoted addition–cyclization and C–C bond cleavage: A convenient synthesis of 2-amino-5-aroylmethylthiazoles derivatives. Org. Biomol. Chem. 2014, 12, 9466–9470. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ni, F.; Shu, W.-M.; Wu, A.-X. Water as an additive for selective synthesis of saturated 1,4-diketones and tetrasubstituted furans directly from 1,4-enediones. Tetrahedron 2014, 70, 6733–6741. [Google Scholar] [CrossRef]
- Shu, W.-M.; Yang, Y.; Zhang, D.-X.; Wu, L.-M.; Zhu, Y.-P.; Yin, G.-D.; Wu, A.-X. Highly Efficient Synthesis of 3a,6a-Dihydrofuro[2,3-b]furans via a Novel Bicyclization. Org. Lett. 2013, 15, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, M.; Wu, L.-M.; Deng, C.; Zhang, D.-X.; Gao, Y.; Zhu, Y.-P.; Wu, A.-X. A facile synthesis of indole-furan conjugates via integration of convergent and linear domino reactions. Tetrahedron 2011, 67, 5142–5149. [Google Scholar] [CrossRef]
- Quast, H.; Seefelder, M.; Ivanova, S.; Heubes, M.; Peters, E.-M.; Peters, K. Tetraacylethenes as Dienophiles and Hetero Dienes in Two-Step Diels–Alder Reactions. Eur. J. Org. Chem. 1999, 3343–3351. [Google Scholar] [CrossRef]
- Adembri, G.; Celli, A.M.; Lampariello, L.R.; Scotton, M.; Sega, A. A new route to highly substituted 1H-pyrrol-3(2H)-ones. Tetrahedron Lett. 1994, 35, 4023–4026. [Google Scholar] [CrossRef]
- Celli, A.M.; Lampariello, L.R.; Scotton, M.; Sega, A. Reaction of 3,4-diacetylethylenes with enamines and Schiff bases. Synthesis of furo-furan and furo-oxazole derivatives. Gazz. Chim. Ital. 1993, 123, 543–547. [Google Scholar] [CrossRef]
- Celli, A.; Scotton, M.; Sega, A. Synthesis of furo-furans by rearrangement of 4-acetylpyrans. Tetrahedron 1992, 48, 5883–5900. [Google Scholar] [CrossRef]
- Adembri, G.; Di Tommaso, A.; Lampariello, L.R.; Scotton, M. Tetraacetylethylene and nitrile oxides: Synthesis of spirofuranisoxazoles. J. Heterocycl. Chem. 1988, 25, 1621–1625. [Google Scholar] [CrossRef]
- Adembri, G.; Anselmi, C.; Celli, A.M.; Lampariello, L.R.; Scotton, M. Facile synthesis of 5-(α-alkyl-substituted) furans. J. Heterocycl. Chem. 1984, 21, 569–571. [Google Scholar] [CrossRef]
- Graziano, M.L.; Iesce, M.R.; Carli, B.; Scarpati, R. Photosensitized Oxidation of Furans; VI. A Simple General Method for the Synthesis of Functionalized cis-1,2-Diacylethylenes (2-Ene-1,4-diones). Synthesis 1983, 125–126. [Google Scholar] [CrossRef]
- Onitsuka, S.; Nishino, H.; Kurosawa, K. Acid-Catalyzed Convenient Transformation of 1-Aryl-2-pentene-1,4-diones into Polyfunctionalized Furans. Tetrahedron Lett. 2000, 41, 3149–3152. [Google Scholar] [CrossRef]
- Onitsuka, S.; Nishino, H. Synthesis of Polyfunctionalized Furans from 1-Aryl-2-pentene-1,4-diones. Tetrahedron 2003, 59, 755–765. [Google Scholar] [CrossRef]
- Kita, Y.; Tohma, H.; Hatanaka, K.; Takada, T.; Fujita, S.; Mitoh, S.; Sakurai, H.; Oka, S. Hypervalent Iodine-Induced Nucleophilic Substitution of para-Substituted Phenol Ethers. Generation of Cation Radicals as Reactive Intermediates. J. Am. Chem. Soc. 1994, 116, 3684–3691. [Google Scholar] [CrossRef]
- Dohi, T.; Murayama, A.; Minamitsuji, Y.; Takenaga, N.; Kita, Y. First hypervalent iodine(III)-catalyzed C–N bond forming reaction: Catalytic spirocyclization of amides to N-fused spirolactams. Chem. Commun. 2007, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- Yakura, T.; Yamauchi, Y.; Tian, Y.; Omoto, M. Catalytic Hypervalent Iodine Oxidation of p-Dialkoxybenzenes to p-Quinones Using 4-Iodophenoxyacetic Acid and Oxone®. Chem. Pharm. Bull. 2008, 56, 1632–1634. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, R.; Nishino, H. Advanced Synthesis of Dihydrofurans. Effect of Formic Acid on the Mn(III)-Based Oxidation. Synth. Commun. 2015, 45, 1807–1816. [Google Scholar] [CrossRef]
- Heiba, E.I.; Dessau, R.M.; Koehl, W.J., Jr. Oxidation by Metal Salts. III. The Reaction of Manganic Acetate with Aromatic Hydrocarbons and the Reactivity of the Carboxymethyl Radical. J. Am. Chem. Soc. 1969, 91, 138–145. [Google Scholar] [CrossRef]
- Nishino, H.; Kurosawa, K. Regioselective Carboxylation of 9-Xanthenones with Manganese(III) Acetate. Bull. Chem. Soc. Jpn. 1983, 56, 474–480. [Google Scholar] [CrossRef]
- Nishino, H.; Kamachi, H.; Baba, H.; Kurosawa, K. Manganese(III)-Mediated Carbon-Carbon Bond Formation in the Reaction of Xanthenes with Active Methylene Compounds. J. Org. Chem. 1992, 57, 3551–3557. [Google Scholar] [CrossRef]
- Nishino, H. Manganese(III)-Based Peroxidation of Alkenes to Heterocycles. In Bioactive Heterocycles I; Topics in Heterocyclic Chemistry; Eguchi, S., Ed.; Springer: Berlin, Germony, 2006; pp. 39–76. [Google Scholar]
- Rahman, M.T.; Haque, M.A.; Igarashi, H.; Nishino, H. Mn(III)-Initiated Facile Oxygenation of Heterocyclic 1,3-Dicarbonyl Compounds. Molecules 2011, 16, 9562–9581. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Miao, D.; Seo, J.G.; Koo, S. Catalytic Oxidation of β-Keto Esters by Manganese(III)/Cobalt(II) and Consecutive Cyclization to Heterocycles. Adv. Synth. Cat. 2014, 356, 3059–3066. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Nishino, H.; Kurosawa, K. Manganese(III)-Based Facile Synthesis of 3-Cyano-4,5-dihydrofurans and 4-Cyano-1,2-dioxan-3-ols Using Alkenes and Acylacetonitrile Building Blocks. Synthesis 1997, 899–908. [Google Scholar] [CrossRef]
- Bhattacharjee, M.N.; Chaudhuri, M.K.; Khathing, D.T. Direct Synthesis of Tris(acetylacetonato)manganese(III). J. Chem. Soc. Dalton Trans. 1982, 669–670. [Google Scholar] [CrossRef]
- Shi, L.-X.; Zhang, V.W.; Wu, C.-D. The synergistic effect of [WZn(VO)2(ZnW9O34)2]12− cores and peripheral metal sites in catalytic oxidative cyclization of acetylacetone. Dalton Trans. 2011, 40, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Kikue, N.; Takahashi, T.; Nishino, H. Mn(III)-Based Oxidative Cyclization of N-Aryl-3-oxobutanamides. Facile Synthesis and Transformation of Substituted Oxindoles. Heterocycles 2015, 90, 540–562. [Google Scholar]
- Kozminykh, E.N.; Goncharov, V.I.; Oborin, D.B.; Kozminykh, V.O. A Simple Method for the Synthesis of Esters of 2-Hydroxy-3-oxo-2,3-dihydrofuran-2-ylacetic Acid. Chem. Heterocycl. Com. 2007, 43, 658–656. [Google Scholar] [CrossRef]
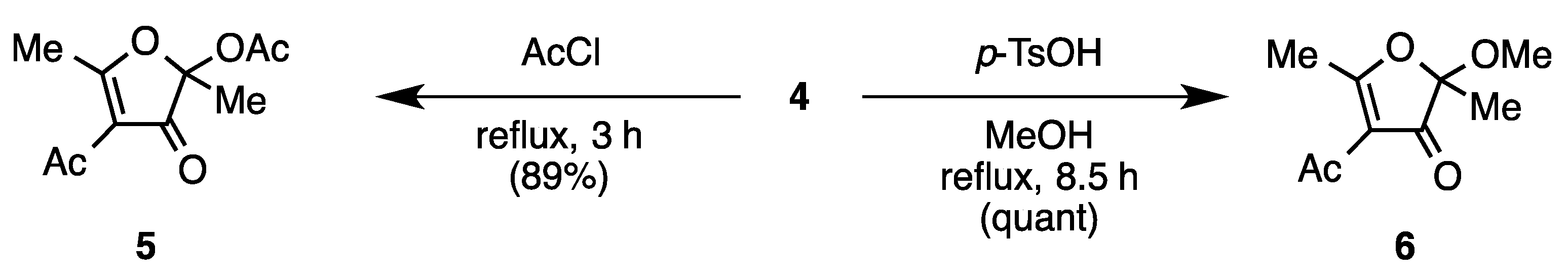
| Entry | Conditions | Additive | Time/h | 4/Yield/% 3 | |
|---|---|---|---|---|---|
| 1 | Mn(acac)3 | argon | 12 | 14 | |
| 2 | air | 12 | 35 | ||
| 3 | dried air stream | 12 | 35 | ||
| 4 | O2 (1 atm) | 12 | 39 | ||
| 5 | O2 (5 atm) | MS4A | 6 | 24 | |
| 6 | air | CF3CH2OH 4 | 12 | 44 | |
| 7 | Mn(OAc)3/Hacac | argon | 18 | 15 | |
| 8 | air | 18 | 29 | ||
| 9 | air | MS4A | 18 | 14 | |
| 10 | air | HCO2H 5 | 3 | 5 | |
| 11 | air | NaOAc 6 | 12 | 21 | |
| 12 | dried air stream | 12 | 52 | ||
| 13 | O2 (1 atm) | CF3CH2OH 4 | 12 | 49 | |
| 14 | air | CF3CH2OH 4 | 12 | 58 | |
| 15 | dry-air stream | CF3CH2OH 4 | 12 | 61 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akazaki, C.; Kawabata, S.; Nishino, H. 4-Acetyl-2-hydroxy-2,5-dimethylfuran-3(2H)-one. Molbank 2016, 2016, M913. https://doi.org/10.3390/M913
Akazaki C, Kawabata S, Nishino H. 4-Acetyl-2-hydroxy-2,5-dimethylfuran-3(2H)-one. Molbank. 2016; 2016(4):M913. https://doi.org/10.3390/M913
Chicago/Turabian StyleAkazaki, Chiaki, Shun Kawabata, and Hiroshi Nishino. 2016. "4-Acetyl-2-hydroxy-2,5-dimethylfuran-3(2H)-one" Molbank 2016, no. 4: M913. https://doi.org/10.3390/M913
APA StyleAkazaki, C., Kawabata, S., & Nishino, H. (2016). 4-Acetyl-2-hydroxy-2,5-dimethylfuran-3(2H)-one. Molbank, 2016(4), M913. https://doi.org/10.3390/M913