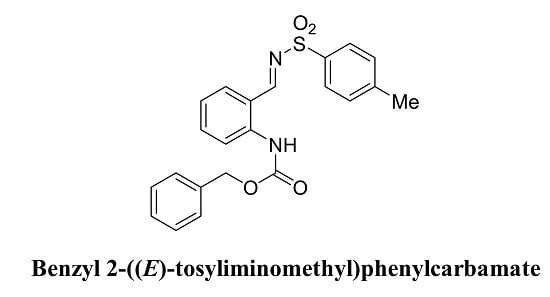
Benzyl 2-((E)-Tosyliminomethyl)phenylcarbamate
Abstract
:1. Introduction
2. Experimental Section
2.1. General Information
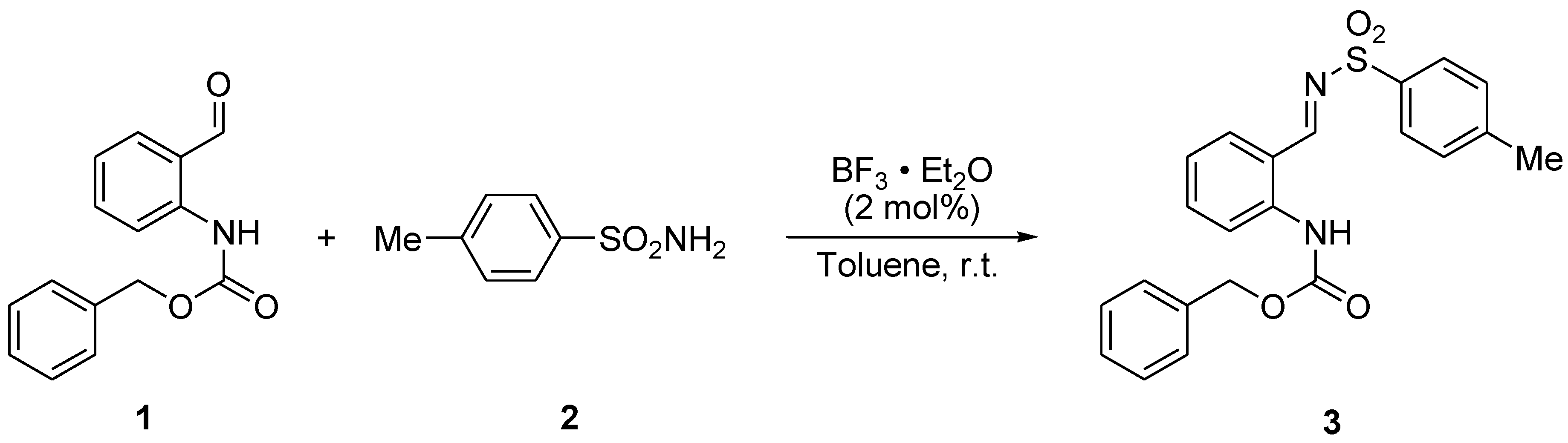
2.2. Syntheis of Benzyl 2-((E)-Tosyliminomethyl)penylcarbamate (3)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff Bases: A short survey on an evergreen chemistry tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.N.; Taploo, C.L. Schiff bases and their applications. J. Sci. Ind. Res. 1982, 41, 501–506. [Google Scholar]
- Zoubi, W.A. Biological Activities of Schiff Bases and Their Complexes: A Review of Recent Works. Int. J. Org. Chem. 2013, 3, 73–95. [Google Scholar] [CrossRef]
- Anand, P.; Patil, V.M.; Sharma, V.K.; Khosa, R.L.; Masand, N. Schiff bases: A review on biological insights. Int. J. Drug Discov. 2012, 3, 851–868. [Google Scholar]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Marins, C.V.B.; de Fátima, A. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Lee, Y.; Heo, S.; Kim, S.-G. Asymmetric one-pot synthesis of 1,4-dihydroquinolines via an organocatalytic aza-Michael/Michael cascade strategy. Adv. Synth. Catal. 2015, 357, 1545–1550. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.-G. One-pot organocatalytic enantioselective Michael addition and aza-cyclization/dehydration cascade reaction strategy: Asymmetric synthesis of highly functionalized 1,4-dihydroquinolines. Tetrahedron Lett. 2015, 56, 4819–4823. [Google Scholar] [CrossRef]
- Yu, M.; Kim, S.-G. Asymmetric organocatalytic Michael addition/aza-cyclization coupled with sequential Michael addition for synthesizing densely polycyclic-fused dihydroquinolines. Tetrahedron Lett. 2015, 56, 4159–4162. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.-G. Asymmetric organocatalytic cascade reaction of aldehydes with 2-amino-β-nitrostyrenes: Synthesis of chiral tetrahydroquinolines and dihydroquinolines. J. Org. Chem. 2014, 79, 8234–8243. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.M.; Knezevic, C.E.; Wall, J.L.; Sun, V.L.; Buss, J.A.; Allen, L.T.; Wenzel, A.G. A copper(II)-catalyzed, sequential Michael-aldol reaction for the preparation of 1,2-dihydroquinolines. Tetrahedron Lett. 2012, 53, 833–836. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, K.M.; Kim, S.-G. Benzyl 2-((E)-Tosyliminomethyl)phenylcarbamate. Molbank 2016, 2016, M912. https://doi.org/10.3390/M912
Ko KM, Kim S-G. Benzyl 2-((E)-Tosyliminomethyl)phenylcarbamate. Molbank. 2016; 2016(4):M912. https://doi.org/10.3390/M912
Chicago/Turabian StyleKo, Kwang Min, and Sung-Gon Kim. 2016. "Benzyl 2-((E)-Tosyliminomethyl)phenylcarbamate" Molbank 2016, no. 4: M912. https://doi.org/10.3390/M912
APA StyleKo, K. M., & Kim, S.-G. (2016). Benzyl 2-((E)-Tosyliminomethyl)phenylcarbamate. Molbank, 2016(4), M912. https://doi.org/10.3390/M912