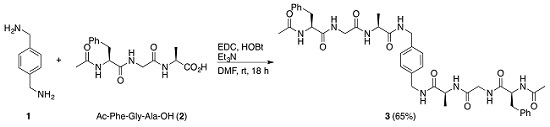
1,4-Bis[(N-acetyl-l-phenylalanyl-glycyl-l-alanyl)aminomethyl]benzene
Abstract
:1. Introduction
2. Experimental Section
2.1. General Information
2.2. Synthesis of 1,4-Bis[(N-acetyl-phenylalanyl-glycyl-alanyl)aminomethyl]benzene (3)
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kemp, D.S.; Li, Z.Q. 2-Amino-2′-carboxydiphenylacetylenes as β-turn mimetics. Synthesis and conformational properties. Tetrahedron Lett. 1995, 36, 4175–4178. [Google Scholar] [CrossRef]
- Yang, X.W.; Yuan, L.H.; Yamamoto, K.; Brown, A.L.; Feng, W.; Furukawa, M.; Zeng, X.C.; Gong, B. Backbone-rigidfied oligo(m-phenylene ethynylenes). J. Am. Chem. Soc. 2004, 126, 3148–3162. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Baldini, L.; Casnati, A.; Chierici, E.; Faimani, G.; Ugozzoli, F.; Ugaro, R. Chiral dimeric capsules from N,C-linked peptidocalix[4]arenes self-assembled through an antiparallel β-sheetlike motif. J. Am. Chem. Soc. 2004, 126, 6204–6205. [Google Scholar] [CrossRef] [PubMed]
- Saraogi, I.; Hamilton, A.D. Recent advances in the development of aryl-based foldamers. Chem. Soc. Rev. 2009, 38, 1726–1743. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Guichard, G. Folding and self-assembly of aromatic and aliphatic urea oligomers: Towards connecting structure and function. Org. Biomol. Chem. 2010, 8, 3101–3117. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.L.; Liu, Y.; Zeng, H.Q. Shape-persistent H-bonded macrocyclic aromatic pentamers. Chem. Commun. 2013, 49, 4127–4144. [Google Scholar] [CrossRef] [PubMed]
- Lingard, H.; Han, J.T.; Thompson, A.L.; Leung, I.K.H.; Scott, R.T.W.; Thompson, S.; Hamilton, A.D. Diphenylacetylene-Linked Peptide Strands Induce Bidirectional β-Sheet Formation. Angew. Chem. Int. Ed. 2014, 53, 3650–3653. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Demizu, Y.; Sato, Y.; Doi, M.; Kurihara, M. Helical foldamer containing a combination of cyclopentane-1,2-diamine and 2,2-dimethylmalonic acid. J. Org. Chem. 2013, 78, 9991–9994. [Google Scholar] [CrossRef] [PubMed]
- Demizu, Y.; Yamashita, H.; Doi, M.; Misawa, T.; Oba, M.; Tanaka, M.; Kurihara, M. Topological study of the structures of heterochiral peptides containing equal amounts of l-Leu and d-Leu. J. Org. Chem. 2015, 80, 8597–8603. [Google Scholar] [CrossRef] [PubMed]
- Horne, W.S.; Gellman, S.H. Foldamers with heterogeneous backbones. Acc. Chem. Res. 2008, 41, 1399–1408. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demizu, Y.; Tsutsui, K.; Misawa, T.; Kurihara, M. 1,4-Bis[(N-acetyl-l-phenylalanyl-glycyl-l-alanyl)aminomethyl]benzene. Molbank 2016, 2016, M893. https://doi.org/10.3390/M893
Demizu Y, Tsutsui K, Misawa T, Kurihara M. 1,4-Bis[(N-acetyl-l-phenylalanyl-glycyl-l-alanyl)aminomethyl]benzene. Molbank. 2016; 2016(2):M893. https://doi.org/10.3390/M893
Chicago/Turabian StyleDemizu, Yosuke, Kohei Tsutsui, Takashi Misawa, and Masaaki Kurihara. 2016. "1,4-Bis[(N-acetyl-l-phenylalanyl-glycyl-l-alanyl)aminomethyl]benzene" Molbank 2016, no. 2: M893. https://doi.org/10.3390/M893
APA StyleDemizu, Y., Tsutsui, K., Misawa, T., & Kurihara, M. (2016). 1,4-Bis[(N-acetyl-l-phenylalanyl-glycyl-l-alanyl)aminomethyl]benzene. Molbank, 2016(2), M893. https://doi.org/10.3390/M893