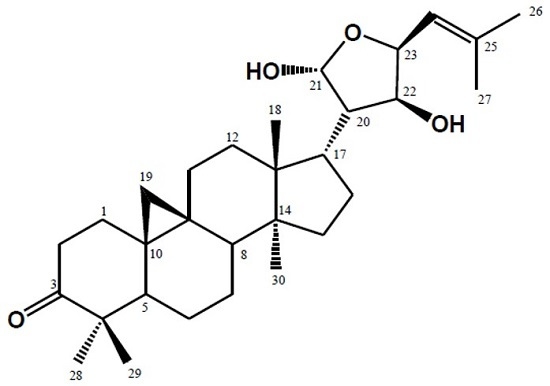
9,19-Cyclolanost-24-en-3-one,21,23-epoxy-21,22-dihydroxy (21R, 22S, 23S) from the Leaves of Lansium domesticum Corr cv Kokossan
Abstract
:1. Introduction
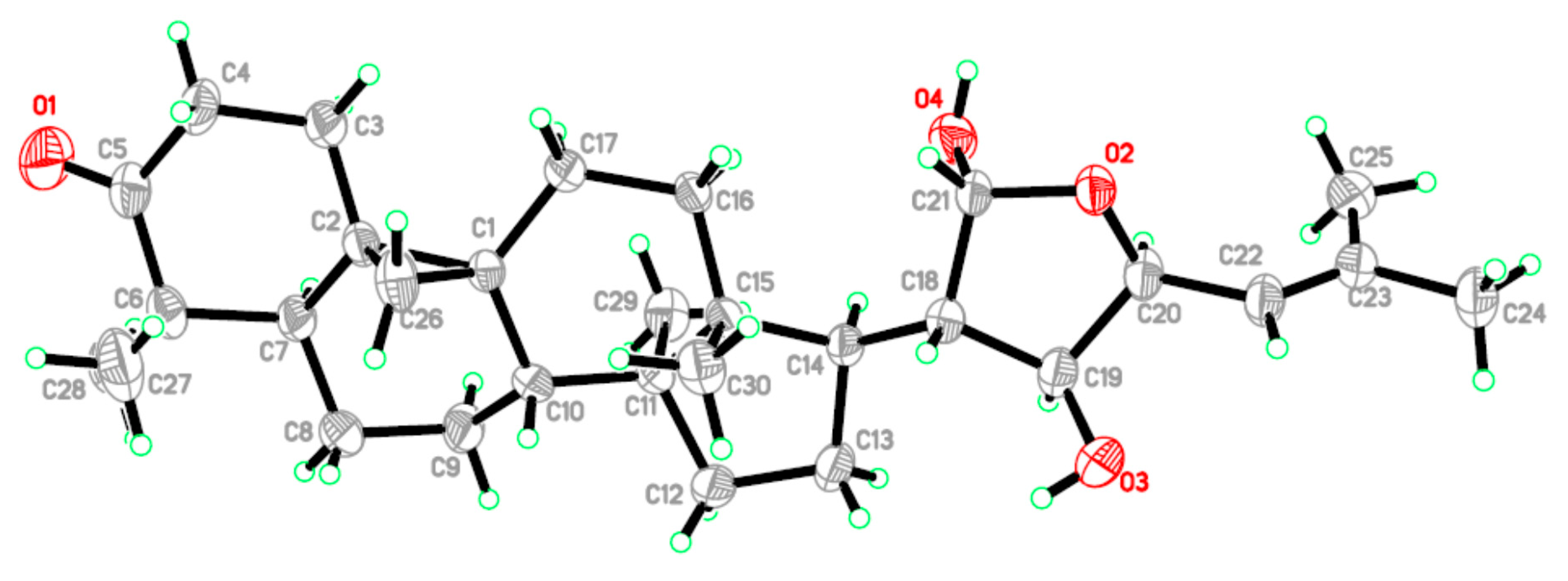
2. Result and Discussion
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Omar, S.; Marcotte, M.; Fields, P.; Sanchez, P.E.; Poveda, L.; Matta, R.; Jimenez, A.; Durst, T.; Zhang, J.; Kinnon, M.; et al. Antifeedant activities of triterpenoids isolated from tropical Rutales. J. Stored Prod. Res. 2007, 43, 92–96. [Google Scholar] [CrossRef]
- Leaman, D.J.; Arnason, J.T.; Yusuf, R.; Sangat-Roemantyo, H.; Soedjito, H.; Angerhofer, C.K.; Pezzuto, J.M. Malaria remedies of the Kenyah of the Apo Kayan, East Kalimantan, Indonesian Borneo: A quantitative assessment of local consensus as an indicator of biological efficacy. J. Ethnopharmacol. 1995, 49, 1–16. [Google Scholar] [CrossRef]
- Nishizawa, M.; Nishide, H.; Hayashi, Y.; Kosela, S. The structure of lansioside A: A novel triterpene glycoside with amino-sugar from Lansium domesticum. Tetrahedron Lett. 1982, 23, 1349–1350. [Google Scholar] [CrossRef]
- Nishizawa, M.; Nishide, H.; Kosela, S.; Hayashi, Y. Structure of lansiosides: Biologically active new triterpene glycosides from Lansium domesticum. J. Org. Chem. 1983, 48, 4462–4466. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishibashi, M.; Fujimoto, H.; Okuyama, E.; Koyano, T.; Kowiyhayakorn, T.; Hayashi, M.; Komiyama, K. New Onoceranoid Constituents from Lansium domesticum. J. Nat. Prod. 2002, 65, 1709–1711. [Google Scholar] [CrossRef] [PubMed]
- Habaguchi, K.; Watanabe, M.; Nakadaira, Y.; Nakanishi, K.; Kaing, A.K.; Lim, F.L. The full structures of lansic acid and its minor congener, an unsymmetric onoceradienedione. Tetrahedron Lett. 1986, 34, 3731–3734. [Google Scholar] [CrossRef]
- Mayanti, T.; Supratman, U.; Mukhtar, M.R.; Awang, K.; Ng, S.W. Kokosanolide from the seed of Lansium domesticum Corr. Acta Crystallogr. 2009, E65, o750. [Google Scholar]
- Supratman, U.; Mayanti, T.; Awang, K.; Mukhtar, M.R.; Ng, S.W. 14-Hydroxy-8,14-secogammacera-7-ene-3,21-dione from the bark of Lansium domesticum Corr. Acta Crystallogr. 2010, E66, o1621. [Google Scholar]
- Manosroi, A.; Jantrawut, P.; Sainakham, M.; Manosroi, W.; Manosroi, J. Anticaner activities oh the extract from longkong (Lansium domesticum) young fruits. Pharm Biol. 2012, 50, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Labrador, P.; Rideout, J.A. Antimicrobial terpenoid from Lansium domesticum. Philipp. Agric. Sci. 2006, 89, 101–105. [Google Scholar]
- Leatemia, J.A.; Isman, M.B. Insecticidal Activity of Crude Seed Extracts of Annona. spp., Lansium domesticum and Sandoricum koetjape Against Lepidopteran Larvae. Phytopatasitica 2004, 32, 30–37. [Google Scholar] [CrossRef]
- Saewan, N.; Sutherland, J.D.; Chantrapromma, K. Antimalarial tetranortriterpenoids from the seed of Lansium domesticum Corr. Phytrochemistry 2006, 67, 2288–2293. [Google Scholar] [CrossRef] [PubMed]
- Tilaar, M.; Wong, W.; Ranti, S.; Wasitaatmadja, M.; Junardy, D. Review of Lansium domesticum Corrêa and its use in cosmetics. Bol. Latinoam. Caribe Plant. Med. Aromat. 2008, 7, 183–189. [Google Scholar]
- Mayanti, T.; Tjokronegoro, R.; Supratman, U.; Mukhtar, M.R.; Awang, K.; Hadi, A.H.A. Antifeedant Triterpenoids from the Seeds and Bark of Lansium domesticum cv Kokossan (Meliaceae). Molecules 2011, 16, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Awang, K.; Loong, X.; Leong, M.F.K.; Supratman, U.; Litaudon, U.; Mukhtar, M.M.; Mohamad, R.K. Triterpenes and steroids from the leaves of Aglaia exima (Meliaceae). Fitoterapia 2012, 83, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Ombuwajo, O.R.; Martin, M.T.; Perromat, G.; Sevenet, T.; Awang, K.; Pais, M. Cytotoxic cycloartantes from Aglaia argentea. Phytochemistry 1996, 41, 1325–1328. [Google Scholar] [CrossRef]


| Position | 13C-NMR | 1H-NMR |
|---|---|---|
| δC ppm (mult., ppm) | δH ppm (Int, mult., J = Hz) | |
| 1 | 33.9 (t) | 1.86 (1H, m) |
| 1.60 (1H, m) | ||
| 2 | 37.8 (t) | 2.21 (1H, ddd, 3.9, 6.5, 10.4) |
| 2.73 (1H, ddd, 6.0, 6.0, 10.4) | ||
| 3 | 215.1 (s) | - |
| 4 | 50.7 (s) | - |
| 5 | 46.7 (d) | 1.93 (1H, m) |
| 6 | 22.1 (t) | 1.59 (1H, m) |
| 1.50 (1H, m) | ||
| 7 | 28.1 (t) | 1.20 (1H, m) |
| 1.86 (1H, m) | ||
| 8 | 48.8 (d) | 1.64 (1H, m) |
| 9 | 21.7 (s) | - |
| 10 | 26.9 (s) | - |
| 11 | 26.7 (t) | 1.93 (1H, m) |
| 1.07 (1H, m) | ||
| 12 | 32.8 (t) | 1.57 (1H, m) |
| 1.64 (1H, m) | ||
| 13 | 48.7 (s) | - |
| 14 | 46.5 (s) | - |
| 15 | 36.3 (t) | 1.05 (1H, m) |
| 1.34 (1H, m) | ||
| 16 | 26.9 (t) | 1.86 (1H, m) |
| 2.00 (1H, m) | ||
| 17 | 49.2 (d) | 1.,57 (1H, m) |
| 18 | 19.4 (q) | 0.97 (1H, s) |
| 19 | 27.1 (t) | 0.70 (1H, d, 3,9) |
| 0.66 (1H, d, 3,9) | ||
| 20 | 59.4 (d) | 2.19 (1H, m) |
| 21 | 101.6 (d) | 5.17 (1H, d, 5.8) |
| 22 | 78.2 (d) | 3.96 (1H, dd, 4.5, 6.5) |
| 23 | 79.0 (d) | 4.68 (1H, dd, 6.5, 8.4) |
| 24 | 123.8 (d) | 5.48 (1H, d, 8,4) |
| 25 | 135.8 (s) | - |
| 26 | 26.1 (q) | 1.73 (3H, s) |
| 27 | 18.4 (q) | 1.66 (3H, s) |
| 28 | 21.1 (q) | 1.09 (3H, s) |
| 29 | 19.7 (q) | 1.16 (3H, s) |
| 30 | 19.4 (q) | 0.96 (3H, s) |
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayanti, T.; Sianturi, J.; Harneti, D.; Darwati; Supratman, U.; Rosli, M.M.; Fun, H.-K. 9,19-Cyclolanost-24-en-3-one,21,23-epoxy-21,22-dihydroxy (21R, 22S, 23S) from the Leaves of Lansium domesticum Corr cv Kokossan. Molbank 2015, 2015, M880. https://doi.org/10.3390/M880
Mayanti T, Sianturi J, Harneti D, Darwati, Supratman U, Rosli MM, Fun H-K. 9,19-Cyclolanost-24-en-3-one,21,23-epoxy-21,22-dihydroxy (21R, 22S, 23S) from the Leaves of Lansium domesticum Corr cv Kokossan. Molbank. 2015; 2015(4):M880. https://doi.org/10.3390/M880
Chicago/Turabian StyleMayanti, Tri, Julinton Sianturi, Desi Harneti, Darwati, Unang Supratman, Mohamad Mustaqim Rosli, and Hoong-Kun Fun. 2015. "9,19-Cyclolanost-24-en-3-one,21,23-epoxy-21,22-dihydroxy (21R, 22S, 23S) from the Leaves of Lansium domesticum Corr cv Kokossan" Molbank 2015, no. 4: M880. https://doi.org/10.3390/M880
APA StyleMayanti, T., Sianturi, J., Harneti, D., Darwati, Supratman, U., Rosli, M. M., & Fun, H.-K. (2015). 9,19-Cyclolanost-24-en-3-one,21,23-epoxy-21,22-dihydroxy (21R, 22S, 23S) from the Leaves of Lansium domesticum Corr cv Kokossan. Molbank, 2015(4), M880. https://doi.org/10.3390/M880