Abstract
Starting from N-methyl-1-{(3S,4S)-4-[2-(trifluoromethyl)phenoxy]-3,4-dihydro-1H-isochromen-3-yl}methanamine (1) target compound 2 is prepared using a mild, direct alkylation approach with 2-fluoroethyl trifluoromethanesulfonate.
Introduction
Since norepinephrine (NE) represents a fundamental neurochemical messenger, its accurate regulation is of major importance. Thus the norepinephrine transporter (NET), responsible for the NE equilibrium in the synaptic cleft, is considered to be involved in a variety of neurological/psychiatric disorders [1,2], but also plays a pivotal role in cardiovascular [1,2,3], and metabolic diseases [3,4,5].
To obtain information about the transporter abundance and density in healthy and pathological living human brains, the most suitable and accurate technique today is positron emission tomography (PET). It represents a non-invasive molecular imaging technique and a suitable approach towards the collection of missing data in the living organism. Moreover, it enables direct quantification of receptor or transporter densities in vivo. However, in order to understand the molecular changes of the noradrenergic system via PET, prior suitable NET-PET radioligands need to be prepared. Non-radioactive reference compounds serve as a basis for preclinical investigations of the respective target.
In this regard, several 3,4-dihydro-1H-isochromene derivatives were synthesized [6,7], which have been described by our research group as potential precursors and reference compounds for PET based investigations of the NET. In continuation of these previous studies, we are reporting in this paper the synthesis of 2-fluoro-N-methyl-N-{[(3S,4S)-4-(2-methylphenoxy)-3,4-dihydro-1H-isochromen-3-yl]methyl}ethanamine (FE@PHOXI2) (3), a reference compound for the NET for future preclinical testing.
Results and Discussion
Derivative 1 was made accessible by reacting 1H-isochromen-4(3H)-one in a Mannich reaction with dimethylamine hydrochloride and paraformaldehyde, followed by reduction of the keto group into an alcohol group with L-Selectride. Subsequent reaction with 1-fluoro-2-(trifluoromethyl)benzene then afforded N,N-dimethyl-1-{(3S,4S)-4-[2-(trifluoromethyl)phenoxy]-3,4-dihydro-1H-isochromen-3-yl}methanamine, which after N-demethylation yielded compound 1.
For the preparation of derivative 2, a mild synthesis approach was chosen, in which freshly prepared 2-fluoroethyl trifluoromethanesulfonate [8] was reacted with the -prior activated-secondary amine (1) at 0 °C (Scheme 1). Due to the high reactivity of triflates, the alkylation was carried out in less than ten minutes while the temperature was slowly raised to 25 °C.
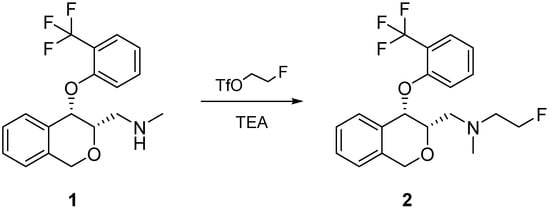
Scheme 1.
Reaction of N-methyl-1-((3S,4S)-4-(2-(trifluoromethyl)phenoxy)-3,4-dihydro-1H-isochromen-3-yl)methanamine to 2-Fluoro-N-methyl-N-(((3S,4S)-4-(2-(trifluoromethyl) phenoxy)-3,4-dihydro-1H-isochromen-3-yl)methyl)ethanamine (FE@PHOXI2).
Experimental Section
General Information
The NMR spectra were recorded from a CDCl3 solution on a Bruker Avance III 400 spectrometer (Billerica, MA, USA) (400 MHz for 1H, 100 MHz for 13C, 40 MHz for 15N, 376 MHz for 19F) at 25 °C. The center of the solvent (residual) signal was used as an internal standard which was related to TMS with δ 7.26 ppm (1H in CDCl3), and δ 77.0 ppm (13C in CDCl3). 19F-NMR spectra were referenced by absolute referencing via Ξ ratio. Digital resolutions were 0.25 Hz/data point in the 1H and 0.3 Hz/data point in the 13C-NMR spectra. Coupling constants (J) are quoted in Hz. The following abbreviations were used to show the multiplicities: s: singlet, d: doublet, t: triplet, q: quadruplet, dd: doublet of doublet, m: multiplet. Mass spectra were obtained on a Shimadzu QP 1000 instrument (Kyoto, Japan, EI, 70 eV), high-resolution mass spectrometry (HRMS) was carried out on a maXis HD ESI-Qq-TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) in the positive-ion mode by direct infusion. Compound purity: all compounds synthesized featured a purity of at least 95%.
2-Fluoro-N-methyl-N-{[(3S,4S)-4-(2-(trifluoromethyl)phenoxy)-3,4-dihydro-1H-isochromen-3-yl]methyl}ethanamine (FE@PHOXI2)
To a suspension of N-methyl-1-((3S,4S)-4-(2-(trifluoromethyl)phenoxy)-3,4-dihydro-1H-isochromen-3-yl)methanamine (0.07 g, 0.22 mmol) in dry ACN (5 mL) was added triethylamine (0.05 mL, 0.33 mmol) under argon atmosphere. After cooling the mixture to 0 °C, 2-fluoroethyl trifluoromethansulfonate (0.03 mL, 0.26 mmol) was slowly added. The solution was stirred for 10 min during which the temperature was allowed to warm to 20 °C. After evaporation of the solvent, the crude reaction product was purified by column chromatography (silica gel 60, 7% MeOH in CH2Cl2).
Yield: 55 mg (65%), light yellow oil.
1H-NMR (400 MHz, CDCl3): δ (ppm) 2.42 (s, 3H, CH2NCH3CH2CH2F), 2.73–2.75 (m, 1H, CH2NCH3CH2CH2F), 2.85 (dd, J = 13.3 Hz and 6.6 Hz, 1H, CH2NCH3CH2CH2F), 2.88–2.91 (m, 1H, CH2NCH3CH2CH2F) 2.98 (dd, J = 13.3 Hz and 6.4 Hz, 1H, CH2NCH3CH2CH2F), 4.12 (dt, J = 2.3 Hz and 6.5 Hz, 1H, isochr 3-CH), 4.46 (t, J = 4.9 Hz, 1H, CH2NCH3CH2CH2F), 4.58 (t, J = 4.9 Hz, 1H, CH2NCH3CH2CH2F), 4.87 (d, J = 15.3 Hz, AB-system, 1H, isochr 1-CH2), 5.05 (d, J = 15.3 Hz, AB-system, 1H, isochr 1-CH2), 5.50 (d, J = 2.3 Hz, 1H, isochr 4-CH), 7.00–7.04 (m, 1H, benz 4-CH), 7.07–7.12 (m, 3H, isochr 8-CH, isochr 5-CH, isochr 6-CH), 7.26–7.30 (m, 1H, isochr 7-CH), 7.35–7.37 (m, 1H, isochr-6-CH), 7.46–7.51 (m, 1H, benz 5-CH), 7.52–7.54 (m, 1H, benz 3-CH).
13C-NMR (100 MHz, CDCl3): δ (ppm) 43.4 (CH2NCH3CH2CH2F), 57.4 (CH2NCH3CH2CH2F), 57.9 (d, J = 19.8 Hz, CH2NCH3CH2CH2F), 67.3 (isochr 1-CH2), 72.7 (isochr 4-CH), 74.6 (isochr 3-CH), 81.8 (d, J = 167.8 Hz, CH2NCH3CH2CH2F), 116.4 (benz 6-CH), 120.6 (q, J = 30.5 Hz, benz 2-C), 120.8 (benz 4-CH), 123.5 (q, J = 272.6 Hz, benz CF3), 124.3 (isochr 8-CH), 126.5 (isochr 6-CH), 127.2 (q, J = 5.3 Hz, benz 3-CH), 128.7 (isochr 7-CH), 128.9 (isochr 5-CH), 131.2 (isochr 4a-C), 132.9 (benz 5-CH), 135.3 (isochr 8a-C), 155.9 (q, J = 1.7 Hz, benz 1-C).
19F-NMR (471 MHz, CDCl3): δ (ppm) −61.8 (s, benz CF), −219.3 (m, CH2NCH3CH2CH2F).
MS: m/z (%) 383 (M+, 1), 350 (1), 322 (1), 218 (1), 175 (1), 132 (4), 115 (3), 104 (4), 90 (100).
HRMS: m/z calculated for C20H22F4NO2 [M+H]+: 384.1581. Found: 384.1532.
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Author Contributions
Catharina Neudorfer: Responsible for the performance of the syntheses and writing; Karem Shanab: Contributions to syntheses and experimental procedures; Wolfgang Holzer: Performance of the NMR analyses; Christina Rami-Mark: Designed parts of the research; Markus Mitterhauser: Designed parts of the research and proofread the manuscript; Wolfgang Wadsak: Designed parts of the research and proofread the manuscript; Helmut Spreitzer: Conceived and supervised the syntheses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, U.; Apparsundram, S.; Galli, A.; Kahlig, K.M.; Savchenko, V.; Schroeter, S.; Quick, M.W.; Blakely, R.D. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J. Neurosci. 2003, 23, 1697–1709. [Google Scholar] [PubMed]
- Kim, C.H.; Hahn, M.K.; Joung, Y.; Anderson, S.L.; Steele, A.H.; Mazei-Robinson, M.S.; Gizer, I.; Teicher, M.H.; Cohen, B.M.; Robertson, D.; et al. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc. Natl. Acad. Sci. USA 2006, 103, 19164–19169. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.K.; Robertson, D.; Blakely, R.D. A mutation in the human norepinephrine transporter gene (SLC6A2) associated with orthostatic intolerance disrupts surface expression of mutant and wild-type transporters. J. Neurosci. 2003, 23, 4470–4478. [Google Scholar] [PubMed]
- Mirbolooki, M.R.; Upadhyay, S.K.; Constantinescu, C.C.; Pan, M.L.; Mukherjee, J. Adrenergic pathway activation enhances brown adipose tissue metabolism: A [18F]FDG PET/CT study in mice. Nucl. Med. Biol. 2014, 41, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Fan, X.; Yeckel, C.W.; Weinzimmer, D.; Mulnix, T.; Gallezot, J.D.; Carson, R.E.; Sherwin, R.S.; Ding, Y.S. Ex vivo and in vivo Evaluation of the Norepinephrine Transporter Ligand [11C]MRB for Brown Adipose Tissue Imaging. Nucl. Med. Biol. 2012, 39, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Neudorfer, C. Development of Novel Precursors and Reference Compounds for PET Based Investigations Targeting the Norepinephrine Transporter and Monoamine Oxidase B. Ph.D. Thesis, University of Vienna, Vienna, Austria, November 2014. [Google Scholar]
- Hudson, S.; Kiankarimi, M.; Eccles, W.; Mostofi, Y.S.; Genicot, M.J.; Dwight, W.; Fleck, B.A.; Gogas, K.; Wade, W.S. Synthesis and structure-activity relationships of selective norepinephrine reuptake inhibitors (sNRI) with a heterocyclic ring constraint. Bioorg. Med. Chem. Lett. 2008, 18, 4495–4498. [Google Scholar] [CrossRef] [PubMed]
- Claremon, D.A.; Zhuang, L.; Leftheris, K.; Tice, C.M.; Xu, Z.; Ye, Y.; Singh, S.B.; Cacatian, S.; Zhao, W.; Himmelsbach, F.; et al. Cyclic Inhibitors of 11beta-Hydroxysteroid Dehydrogenase 1. PCT Int. Appl. WO 2009134392 A1, November 2009. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).