Abstract
The title compound (2) was prepared in high yield by catalytic transfer hydrogenation of the 6-nitro precursor (1), using hydrazine hydrate as the hydrogen source. Under the conditions employed, the ester groups remain unaffected. The new compound was fully characterised by elemental analysis, 1H-NMR, 13C-NMR, MS and HRMS data.
1-Methylcarbazole constitutes an essential fragment of the antitumor alkaloid, olivacine (1,5-dimethylpyrido[3,4-b]carbazole) [1] and drug candidates derived thereof, like S16020-2 [2]. Such compounds are known to act as topoisomerase II poisons and as another common structural feature they possess a heteroaromatic ring fused to the carbazole 2,3-bond. Typically, this is a pyridine unit (like in olivacine and its isomer, ellipticine [3]), but there are also examples for various diazine-fused 1-methyl- and 1,4-dimethylcarbazoles, exhibiting antitumor activity [4]. Among them, pyridazino[4,5-b]carbazoles (representing a 3-aza-olivacine scaffold) have been reported by us [5,6,7,8] and others [9]. As a key intermediate for the synthesis of these tetracycles, 1-methylcarbazole-2,3-dicarboxylic esters have been used and the latter were found to provide a convenient access also to some cytotoxic 4-methylpyrrolo[3,4-b]carbazole-1,3(2H,5H)-diones [10], which again feature the 1-methylcarbazole motif. In the course of extensive structural variation of the A-ring substitution pattern of such b-fused 1-methylcarbazoles, we recently reported the synthesis and cyclisation reactions of some halo- and nitro-substituted 1-methylcarbazole-2,3-dicarboxylic acid dimethyl esters [11]. As a further extension of our building-block library, we here describe the convenient preparation of dimethyl 6-amino-1-methyl-9H-carbazole-2,3-dicarboxylate (2) from the corresponding 6-nitro compound (1) [11] by a catalytic transfer hydrogenation protocol.
Whereas catalytic hydrogenation with gaseous hydrogen is a well-established method also for the reduction of nitrocarbazoles into aminocarbazoles (see, for example [12,13]), employment of a solid or liquid hydrogen source has the advantage of safer handling and easier reaction monitoring, especially if elevated temperatures are required. Such protocols, known as catalytic transfer hydrogenation have also been used in the nitrocarbazole series, and the combination of palladium/carbon as catalyst and hydrazine hydrate as hydrogen source had been found to be particularly useful for this purpose [14], as the yields are generally high and there are no non-volatile reagents or by-products involved. On the other hand, hydrazine hydrate is a strong nucleophile and it is known to react with carbazole-2,3-dicarboxylic acid esters at elevated temperature to give either carboxylic hydrazides [9] or (after intramolecular cyclisation) carbazole-fused pyridazinediones [5,9]. We now found that heating (reflux, 30 min) of the nitrocarbazole diester 1 with excess hydrazine hydrate in methanolic solution, using 10% palladium/carbon as catalyst leads to a clean reduction of the nitro group into the amino function, leaving the ester groups completely unaffected (Scheme 1). Thus, the title compound (2) was isolated in 88% yield in high purity simply by filtering off the catalyst and subsequent evaporation of the reaction solution, followed by recrystallisation.
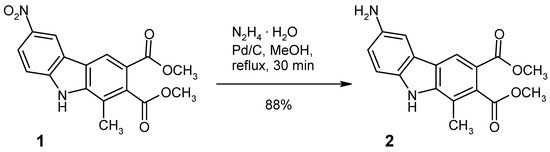
Scheme 1.
Synthesis of dimethyl 6-amino-1-methyl-9H-carbazole-2,3-dicarboxylate (2).
The amino compound (2) was fully characterized by elemental analysis (indicating partial hydration), 1H-NMR, 13C-NMR, IR, MS and HRMS. Expectedly, the transformation of the nitro group into an amino function is associated with a significant high-field shift of the 5-H and 7-H resonances in the 1H-NMR spectrum, the NH2 group appears as a broad singulet at 4.84 ppm. The IR spectrum shows a new, strong absorption band at 3404 cm–1 and the mass spectrum (EI) exhibits the molecular ion at m/z = 312 as the base peak.
Experimental Section
The melting point was determined on a Kofler hot-stage microscope (Reichert, Vienna, Austria) and is uncorrected. The IR spectrum was recorded on a Perkin-Elmer Spectrum 1000 instrument (London, UK), 1H-NMR and 13C-NMR spectra were recorded on a Bruker Avance III 400 spectrometer (Karlsruhe, Germany). The low-resolution mass spectrum was obtained on a Shimadzu QP5050A DI 50 instrument (Kyoto, Japan), the HRMS was recorded on a Bruker maXis HD QTOF system.
Dimethyl 6-Amino-1-methyl-9H-carbazole-2,3-dicarboxylate (2)
To a solution of dimethyl 1-methyl-6-nitro-9H-carbazole-2,3-dicarboxylate [11] (1) (171 mg, 0.5 mmol) and 98% hydrazine monohydrate (510 mg, 10 mmol) in MeOH (30 mL) was added 10% Pd/C (20 mg), and the mixture was refluxed for 30 min. The catalyst was removed by filtration and rinsed carefully with MeOH. The combined filtrate and washings were evaporated to dryness under reduced pressure. The residue was suspended in water (10 mL), stirred for 10 min and filtered off again. Recrystallisation of this material from MeOH/water afforded 137 mg (88%) of the title compound (2) as yellow crystals, mp 239–240 °C.
IR (KBr): νmax (cm−1): 3404, 3304, 3216, 2948, 1712, 1696, 1500, 1432, 1356, 1272, 1256, 1200, 1098, 1018, 810, 784, 612.
MS (EI, 70 eV): m/z = 312 (M+, 100%), 281 (26), 266 (47), 265 (44), 194 (40), 141 (28).
1H-NMR (DMSO-d6, 400 MHz): δ = 11.35 (s, 1H, carbazole NH), 8.42 (s, 1H, 4-H), 7.30 (d, J = 8.5 Hz, 1H, 8-H), 7.29 (d, J = 2.0 Hz, 1H, 5-H), 6.86 (dd, J = 8.7, 2.0 Hz, 1H, 7-H), 4.84 (br s, 2H, NH2), 3.84 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 2.42 (s, 3H, 1-CH3).
13C-NMR (DMSO-d6, 100 MHz): δ = 169.5, 166.4, 142.6, 141.3, 133.6, 131.1, 123.2, 121.2, 120.3, 117.4, 116.6, 116.4, 112.0, 103.7, 52.2, 52.0, 13.9.
Elemental Analysis: Calcd. for C17H16N2O4·0.15H2O: C, 64.82%; H, 5.22%; N, 8.89%. Found: C, 64.75%; H, 5.00%; N, 8.85%.
HRMS Calcd. for C17H17N2NaO4 ([M+Na]+): 335.1002. Found: 335.1003.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Author Contributions
Katharina Tropper: Experimental synthetic work, literature search; Norbert Haider: Synthesis planning, literature search, NMR recording and interpretation.
Conflicts of Interest
The corresponding author is the Editor-in-Chief of this journal. Manuscript handling, including submission to the regular peer-review process, was transferred to the publisher.
References
- Schmutz, J.; Hunziker, H. Die alkaloide von Aspidosperma olivaceum. Pharm. Acta Helv. 1958, 33, 341–347. [Google Scholar] [PubMed]
- Le Mée, S.; Pierré, A.; Markovits, J.; Atassi, G.; Jacquemin-Sablon, A.; Saucier, J.-M. S16020-2, a new highly cytotoxic antitumor olivacine derivative: DNA interaction and DNA topoisomerase II inhibition. Mol. Pharmacol. 1998, 53, 213–220. [Google Scholar] [PubMed]
- Miller, C.M.; McCarthy, F.O. Isolation, biological activity and synthesis of the natural product ellipticine and related pyridocarbazoles. RSC Adv. 2012, 2, 8883–8918. [Google Scholar] [CrossRef]
- Haider, N. Diazine analogues of the pyridocarbazole alkaloids. Curr. Org. Chem. 2006, 10, 363–375. [Google Scholar] [CrossRef]
- Haider, N.; Jbara, R.; Khadami, F.; Wanko, R. Synthesis of pyridazino[4,5-b]carbazoles as potential antitumor agents. Heterocycles 1998, 48, 1609–1622. [Google Scholar] [CrossRef]
- Haider, N.; Käferböck, J.; Mátyus, P. Diels-Alder reaction of pyrano[3,4-b]indolones with an electron-deficient pyridazinone: A new pathway to carbazole-fused pyridazines. Heterocycles 1999, 51, 2703–2710. [Google Scholar] [CrossRef]
- Haider, N.; Sotelo, E. 1,5-Dimethyl-6H-pyridazino[4,5-b]carbazole, a 3-aza bioisoster of the antitumor alkaloid olivacine. Chem. Pharm. Bull. 2002, 50, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Käferböck, J. Intramolecular [4+2] cycloaddition reactions of indolylalkylpyridazines: Synthesis of annulated carbazoles. Tetrahedron 2004, 60, 6495–6507. [Google Scholar] [CrossRef]
- Landelle, H.; LaDuree, D.; Cugnon de Sevricourt, M.; Robba, M. Synthèse et etude pharmacologique de pyridazino[4,5-b]carbazoles. Chem. Pharm. Bull. 1989, 37, 2679–2682. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Jbara, R.; Käferböck, J.; Traar, U. Synthesis of tetra- and pentacyclic carbazole-fused imides as potential antitumor agents. ARKIVOC 2009, 38–47. [Google Scholar] [CrossRef]
- Haider, N.; Marian, B.; Nagel, T.; Tarnai, M.; Tropper, K. Electrophilic substitution of dimethyl 1-methylcarbazole-2,3-dicarboxylate: Synthesis of new b-Fused carbazoles as potential antitumor agents. J. Braz. Chem. Soc. 2014, 25, 1965–1974. [Google Scholar] [CrossRef]
- Block, M.H.; Boyer, S.; Brailsford, W.; Brittain, D.R.; Carroll, D.; Chapman, S.; Clarke, D.S.; Donald, C.S.; Foote, K.M.; Godfrey, L.; et al. Discovery and optimization of a series of carbazole ureas as NPY5 antagonists for the treatment of obesity. J. Med. Chem. 2002, 45, 3509–3523. [Google Scholar] [CrossRef] [PubMed]
- Hudkins, R.L.; Zulli, A.L.; Reddy, D.R.; Gingrich, D.E.; Tao, M.; Becknell, N.C.; Diebold, J.L.; Underiner, T.L. Preparation of novel fused pyrrolocarbazoles for treating or preventing angiogenesis and angiogenic disorders. U.S. Patent 2,005,143,442, 30 June 2005. [Google Scholar]
- Deady, L.W.; Sette, R.M. Lithiation of pivaloylamino derivatives of dibenzofuran and 9-methylcarbazole. Aust. J. Chem. 2001, 54, 177–180. [Google Scholar] [CrossRef]
© 2015 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).