Abstract
Ethyl 4,4''-Dibromo-5'-(butylamino)-2',6'-dinitro-[1,1':3',1''-terphenyl]-4'-carboxylate (2) which is a m-terphenyl derivative containing an hexasubstituted, highly functionalized substituted benzene core, has been synthesized.
Introduction
Multifunctionalized benzene derivatives are important frameworks in several areas. Particularly, arylamine derivatives are important as molecular components in electronic devices and dyes [1]. Terphenyl and polyarylamine cores have attracted attention as organic components for semiconductor materials because of their high thermal stability and excellent optic properties due to the broken conjugation. Poly(m-phenylene) derivatives are particularly interesting in some aspects of materials science, such as artificial helical polymers [2] and dye-sensitized solar cells [3,4,5], and also in supramolecular chemistry [6]. Additionally, some terphenyl-derived compounds have interesting pharmacological properties [7] and these compounds have also been used as efficient ligands for metal-catalyzed reactions [8,9,10]. Due to their importance, many research groups have focused their efforts in the development of efficient methods for the synthesis of polysubstituted and highly functionalized benzene derivatives from simple acyclic substrates [11,12,13].
We describe here the preparation of a m-terphenyl derivative (2) containing an hexasubstituted central ring that, besides the two aryl-aryl bonds, contains four functional groups, namely one ester, one alkylamino and two nitro functions. This pattern potentially allows transformations of interest in materials chemistry, such as the preparation of carbazole systems via the Cadogan reaction. Furthermore, the presence of the two 4-bromo substituents in the peripheral aromatic rings allows to perform polymerizations via cross-coupling reactions.
The synthesis of our target compound started from precursor 1, which was in turn obtained in 77% overall yield by a microwave-assisted three-component reaction between butylamine, ethyl 3-oxobutanoate, and 4,4'-dibromochalcone [14], followed by dehydrogenation with DDQ in toluene [15,16]. Compound 1 was regioselectively nitrated in its central benzene ring in almost quantitative yield by treatment with sulfonitric mixture in acetic acid at 0 °C, followed by heating at 50 °C for 40 minutes (Scheme 1).
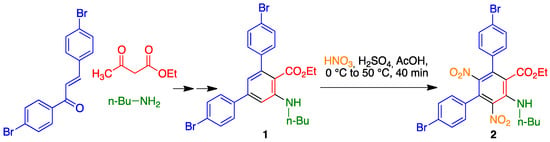
Scheme 1.
Synthesis of ethyl 4,4''-dibromo-5'-(butylamino)-2',6'-dinitro-[1,1':3',1''-terphenyl]-4'-carboxylate.
Ab initio calculations at the 6-31+G* level showed that, not unexpectedly, steric congestion around the central benzene ring drove several of the functional groups out of conjugation (Figure 1). This is an interesting feature, as it is expected to increase the reactivity of compound 2 and thereby render it more amenable to synthetic manipulation aimed at its use as a building block in the synthesis of new organic materials.
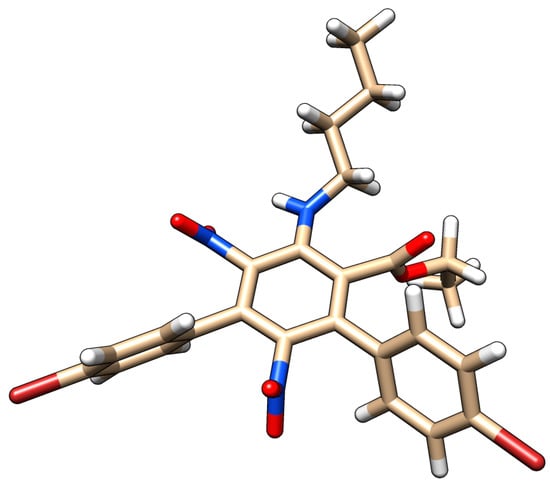
Figure 1.
Three-dimensional structure of compound 2, minimized at the 6-31+G* level.
Experimental Section
Melting points were measured in an open capillary tube and are uncorrected. 1H-NMR and 13C-NMR spectra were recorded on a Bruker (Avance) 250 MHz NMR instrument (Billerica, MA, USA) maintained by the CAI de Resonancia Magnética, Universidad Complutense, using CDCl3 as solvent and the residual CHCl3 as reference. Chemical shifts are given in parts per million (δ scale) and the coupling constants are given in Hertz. Silica gel-G plates (Merck) were used for TLC analysis. Elemental analyses were measured by the CAI de Microanálisis Elemental, Universidad Complutense, on a Leco 932 CHNS analyser (St. Joseph, MI, USA). IR spectra were recorded on an Agilent CARY630 FTIR instrument (neat sample on a NaCl window) (Santa Clara, CA, USA). The computational studies were performed with MacSpartan 10 software.
Ethyl 4,4''-Dibromo-5'-(butylamino)-2',6'-dinitro-[1,1':3',1''-terphenyl]-4'-carboxylate (2). A solution of ethyl-6-butylamino-2,4-di-(4-bromophenyl)-benzoate 1 (425 mg, 0.8 mmol) in acetic acid (2 mL) was added dropwise in an ice bath to 5 mL of a stirred nitrating mixture, previously prepared by mixing nitric acid and sulfuric acid (1/1). The reaction was heated at 50 °C for 40 minutes and upon completion of the reaction, as indicated by TLC (mobile phase, 9:1 petroleum ether–ethyl acetate, Rf of compound 2, 0.47), the reaction mixture was cooled to room temperature and poured onto an ice bath. The precipitate obtained was isolated by filtration under vacuum and was crystalized with ethanol to give 482 mg (97 % yield) of compound 2 as pale yellow crystals.
Melting point: 180–181 °C.
IR νmax (film): 2960, 2875, 1734, 1541 cm−1.
1H-NMR (250 MHz, CDCl3) δ 7.70–7.55 (m, 4H), 7.28–7.22 (m, 4H), 4.18–3.92 (m, 3H), 3.85–3.66 (m, 1H), 1.82–1.53 (m, 2H), 1.46–1.23 (m, 2H), 1.08–0.82 (m, 6H).
13C-NMR (63 MHz, CDCl3) δ 162.7, 151.2, 148.5, 135.7, 135.1, 132.6, 132.2, 130.4, 130.2, 130.1, 129.0, 126.6, 125.9, 125.1, 63.5, 55.0, 28.9, 20.0, 13.6, 13.6.
Elemental analysis (%) calcd for C25H23Br2N3O6: C, 48.33; H, 3.73; N, 6.76; found: C, 48.28; H, 3.80; N, 6.68.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Financial support from Ministerio de Economía y Competitividad (MINECO) is gratefully acknowledged (grant CTQ-2012-33272-BQU).
Author Contributions
D.R. performed the experimental work and product characterization. J.F.G. and J.C.M. prepared the manuscript, with contributions from all authors. The overall project management was done by J.C.M.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Kapustin, D.; Prostyakova, A.; Bryk, Y.; Yagudaeva, E.; Zubov, V. New Composite Materials Modified with Nano-Layers of Functionalized Polymers for Bioanalysis and Medical Diagnostics. In Nanocomposites and Polymers with Analytical Methods; Cupoletti, J., Ed.; InTech: Rijeka, Croatia, 2011; Chapter 4. [Google Scholar]
- Yashima, E.; Maeda, K.; Furusho, Y. Single- and Double-Stranded Helical Polymers: Synthesis, Structures, and Functions. Acc. Chem. Res. 2008, 41, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.-S.; Chen, W.-T.; Hsu, C.-Y.; Chou, H.-H.; Lin, J.T.; Yeh, M.-C.P. Arylamine-Based Dyes for p-Type Dye-Sensitized Solar Cells. Org. Lett. 2011, 13, 4930–4933. [Google Scholar] [CrossRef] [PubMed]
- Sasabe, H.; Seino, Y.; Kimura, M.; Kido, A. m-Terphenyl-Modifed Sulfone Derivative as a Host Material for High-Efficiency Blue and Green Phosphorescent OLEDs. J. Chem. Mater. 2012, 24, 1404–1406. [Google Scholar] [CrossRef]
- Kim, D.; Coropceanu, V.; Brédas, J.-L. Design of Efficient Ambipolar Host Materials for Organic Blue Electrophosphorescence: Theoretical Characterization of Hosts Based on Carbazole Derivatives. J. Am. Chem. Soc. 2011, 133, 17895–17900. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, Y.; Crump, M.P.; Davis, A.P. A Synthetic Lectin Analog for Biomimetic Disaccharide Recognition. Science 2007, 318, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, P.; Padmanabhan, R.; Rajesh, N. Synthesis, Study on Anti-Arthritic, Anti-Inflammatory Activity and Toxicity of Some Novel Bis-Oxy Cyclophane Diamides. Bioorg. Med. Chem. Lett. 2012, 22, 3770–3775. [Google Scholar] [CrossRef] [PubMed]
- Kays, D.L. Recent Developments in Transition Metal Diaryl Chemistry. Dalton Trans. 2011, 40, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Kays, D.L. The Stabilization of Organometallic Complexes Using m-Terphenyl Ligands. Organomet. Chem. 2010, 36, 56–76. [Google Scholar]
- Clyburne, J.A.C.; McMullen, N. Unusual Structures of Main Group Organometallic Compounds Containing m-Terphenyl Ligands. Coord. Chem. Rev. 2000, 210, 73–99. [Google Scholar] [CrossRef]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-Aryl Bond Formation One Century After the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1469. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, J.; Wulff, W.D.; Rheingold, A.L. The First Examples of a Meta-Benzannulation from the Reaction of Fischer Carbene Complexes with Alkynes. J. Am. Chem. Soc. 2003, 125, 8980–8981. [Google Scholar] [CrossRef] [PubMed]
- Odedra, A.; Wu, C.-J.; Pratap, T.B.; Huang, C.-W.; Ran, Y.-F.; Liu, R.-S. Ruthenium-Catalyzed Aromatization of Enediynes via Highly Regioselective Nucleophilic Additions on a π-Alkyne Functionality. A Useful Method for the Synthesis of Functionalized Benzene Derivatives. J. Am. Chem. Soc. 2005, 127, 3406–3412. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, D.; González, J.F.; Menéndez, J.C. Montmorillonite Clay-Promoted, Solvent-Free Cross-Aldol Condensations under Focused Microwave Irradiation. Molecules 2014, 19, 7317–7326. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Menéndez, J.C. Two-Step Stereocontrolled Synthesis of Densely Functionalized Cyclic β-Aminoesters Containing Four Stereocenters, Based on a New Cerium(IV) Ammonium Nitrate Catalyzed Sequential Three-Component Reaction. Org. Lett. 2008, 10, 4303–4306. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, D.; González, J.F.; Menéndez, J.C. Microwave-Assisted, Sequential Four-Component Synthesis of Polysubstituted 5,6-Dihydroquinazolinones from Acyclic Precursors and a Mild, Halogenation-Initiated Method for Their Aromatization Under Focused Microwave Irradiation. Green Chem. 2013, 15, 511–517. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).