Abstract
The title indole-based compound that enforces tripodal topology and is potential applicable for the use as artificial receptor, was prepared by a simple reaction of 1,3,5-benzenetricarbonyl trichloride with 5-methoxytryptamine. The compound was characterized by elemental analysis, 1H-NMR, 13C-NMR and mass spectrometry.
Introduction
The indole group was found to be a valuable building block for the construction of artificial receptors, which are able to bind both ionic [1,2,3] and neutral substrates, like some carbohydrates [4,5]. The design of indole-based carbohydrate receptors [6,7,8,9,10] was inspired by the binding modes of carbohydrate-binding proteins, which often use indole (Trp) and/or imidazole (His) groups to bind the carbohydrate substrate by hydrogen bonding and CH-π interactions [11].
In this paper we describe the simple synthesis of the methoxyindole-based compound 3 that enforces tripodal topology and is potential applicable for the use as artificial receptor. The synthesis of 3 involves the reaction of 1,3,5-benzenetricarbonyl trichloride (1) with 5-methoxytryptamine (2) (Scheme 1). A tripodal analogue of compound 3, lacking the methoxy substituents, was previously reported as an anion receptor, showing preference for hydrogen sulfate over other anions [12]. In the case of 3, however, molecular modeling calculations indicated the ability of this compound to act as receptor for cations, such as NH4+ ion and other organic ammonium ions [13]. The complex with NH4+ ion can be stabilized by the formation of hydrogen bonding interactions with the methoxy groups of 3, NH-π interactions with the indole rings as well as NH-π interaction with the central benzene ring of 3 (example of an energy-minimized structure of the 1:1 complex between 3 and NH4+ is shown in Figure 1). Proton NMR titration technique was employed to prove this suggestion. 1H-NMR spectroscopic titrations of compound 3 with NH4PF6 in CD3CN revealed movements of the signals of 3 (for example, upfield shifts of the benzene CH and amide NH of 3) and provided indications for complex formation between the two binding partners. Titration data were analyzed using the WinEQNMR 2 program [14] and gave good fit only to the mixed 1:1 and 2:1 receptor-substrate binding model (K11 = 370 M−1, K21 = 890 M−1; average values from three titrations); the formation of complexes with 2:1 stoichiometry was further indicated by the mole ratio plot (see Figure S5).
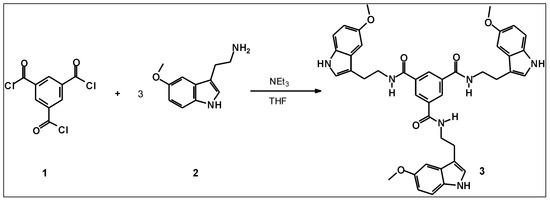
Scheme 1.
Synthesis of the title compound N,N′,N″-Tris[(5-methoxy-1H-indol-3-yl)ethyl]benzene-1,3,5-tricarboxamide (3).
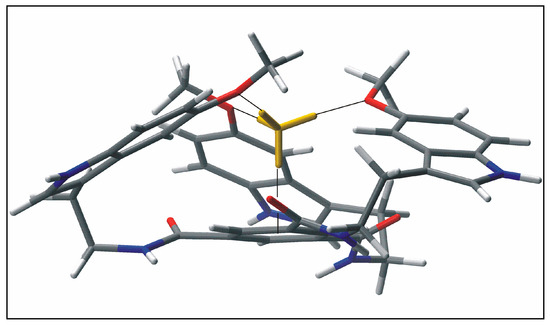
Figure 1.
Energy-minimized structure of the 1:1 complex between 3 and NH4+ (MacroModel V.8.5, OPLS 2001 force field, MCMM, 50000 steps). Color code: receptor N, blue; O, red; C, gray; NH4+ is highlighted in yellow.
Experimental Section
1,3,5-Benzenetricarbonyl trichloride (1) (110 mg, 0.41 mmol) was dissolved in CH2Cl2/THF (3 mL/7 mL) and added dropwise to a mixture of 5-methoxytryptamine (2) (355 mg, 1.87 mmol) and triethylamine (0.35 mL, 252 mg, 2.49 mmol) in dry THF (20 mL). The reaction mixture was stirred for 48 h at room temperature. After addition of an additional amount of THF (20 mL), the solution was allowed to stand at room temperature and the formed precipitate, involving 5-methoxytryptamine and triethylamine hydrochlorides, was separated by filtration. Then, water (20 mL) was added, the mixture stirred for 60 minutes at room temperature and the organic solvents were removed under reduced pressure. The crude product was separated from the aqueous phase, dissolved in THF and dried over MgSO4. The solvent was evaporated and the residue purified by column chromatography (silica gel, CHCl3/CH3OH, 15:1, v/v). The product 3 was obtained as a white solid in 76% yield.
230 mg (0.32 mmol, 76%).
Mp = 122–123 °C.
Rf = 0.45 (silica gel, methanol/chloroform 1:15 v/v).
Rf = 0.57 (silica gel, methanol/chloroform 1:7 v/v).
1H-NMR (400 MHz, THF-d8): δ [ppm] = 3.01 (t, J = 7.3 Hz, 6H, CH2), 3.67 (m, 6H, CH2), 3.75 (s, 9H, OCH3), 6.70 (dd, J = 8.7/2.4 Hz, 3H, Haryl), 7.02 (d, J = 2.4 Hz, 3H, Haryl), 7.11 (d, J = 2.4 Hz, 3H, Haryl), 7.15 (d, J = 8.7 Hz, 3H, Haryl), 8.09 (t, J = 5.8 Hz, 3H, NH), 8.43 (s, 3H, Haryl), 9.78 (s, 3H, NH).
1H-NMR (500 MHz, CD3CN, [3] = 1 mM): δ [ppm] = 3.03 (t, J = 7.0 Hz, 6H, CH2), 3.68 (m, 6H, CH2), 3.77 (s, 9H, OCH3), 6.79 (dd, J = 8.8 Hz/2.4 Hz, 3H, Haryl), 7.12 (m, 6H, Haryl), 7.24 (t, J = 5.4 Hz, 3H, NH), 7.30 (d, J = 8.8 Hz, 3H, Haryl), 8.24 (s, 3H, Haryl), 8.96 (s, 3H, NH).
13C-NMR (100 MHz, THF-d8): δ [ppm] = 26.6, 41.6, 55.8, 101.0, 112.4 (2C), 113.4, 123.7, 129.0 (2C), 133.1, 136.7, 154.8, 166.4.
HRMS (ESI) calcd. for C42H43N6O6 [M + H]+ 727.323860. Found 727.323837.
Elemental Analysis: calcd. for C42H42N6O6: C, 69.40; H, 5.82; N, 11.57. Found: C, 69.33; H, 6.01; N, 11.34.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4References and Notes
- For examples, see: Kubik, S. Anion recognition in water. Chem. Soc. Rev. 2010, 39, 3648–3663. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A. Anion receptor chemistry: highlights from 2008 and 2009. Chem. Soc. Rev. 2010, 39, 3746–3771. [Google Scholar] [CrossRef] [PubMed]
- Kataev, E.A.; Müller, C. Recent advances in molecular recognition in water: artificial receptors and supramolecular catalysis. Tetrahedron 2014, 70, 137–167. [Google Scholar]
- Mazik, M. Molecular recognition of carbohydrates by acyclic receptors employing noncovalent interactions. Chem. Soc. Rev. 2009, 38, 935–956. [Google Scholar] [CrossRef] [PubMed]
- Mazik, M. Recent developments in the molecular recognition of carbohydrates by artificial receptors. RSC Adv. 2012, 2, 2630–2642. [Google Scholar] [CrossRef]
- Mazik, M.; Kuschel, M. Highly effective acyclic carbohydrate receptors consisting of aminopyridine, imidazole, and indole recognition units. Chem. Eur. J. 2008, 14, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Mazik, M.; Hartmann, A. Recognition properties of receptors consisting of imidazole and indole recognition units towards carbohydrates. Beilstein J. Org. Chem. 2010, 6. No 9. [Google Scholar] [CrossRef]
- Sonnenberg, C.; Hartmann, A.; Mazik, M. Molecular recognition of carbohydrates: Evaluation of the binding properties of pyrazole-based receptors and their comparison with imidazole- and indole-based systems. Nat. Prod. Commun. 2012, 7, 321–326. [Google Scholar] [PubMed]
- Rosien, J.-R.; Seichter, W.; Mazik, M. Trimethoxybenzene- and trimethylbenzene-based compounds bearing imidazole, indole and pyrrole groups as recognition units: Synthesis and evaluation of the binding properties towards carbohydrates. Org. Biomol. Chem. 2013, 11, 6569–6579. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.; Rosien, J.-R.; Mazik, M. Synthesis of Compounds Based on a Dimesitylmethane Scaffold and Representative Binding Studies Showing Di- vs. Monosaccharide Preference. Tetrahedron 2014, 70, 8758–8767. [Google Scholar] [CrossRef]
- For examples, see: Lis, H.; Sharon, N. Lectins; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Ito, K.; Umetsu, T.; Kamimura, A. Indole- and amide-based tripodal anion receptor: High affinity towards a hydrogen sulfate. Trends Heterocycl. Chem. 2008, 13, 69–73. [Google Scholar]
- For a review on recognition of ammonium ions, see: Späth, A.; König, B. Molecular recognition of organic ammonium ions in solution using synthetic receptors. Beilstein J. Org. Chem. 2010, 6. No 32. [Google Scholar]
- Hynes, M.J. EQNMR: A computer program for the calculation of stability constants from nuclear magnetic resonance chemical shift data. J. Chem. Soc. Dalton Trans. 1993, 311–312. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).